当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Autocatalytic Carbonyl Arylation through In Situ Release of Aryl Nucleophiles from N-Aryl-N'-Silyldiazenes.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-02-06 , DOI: 10.1002/anie.201916004 Clément Chauvier 1 , Lucie Finck 1 , Elisabeth Irran 1 , Martin Oestreich 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-02-06 , DOI: 10.1002/anie.201916004 Clément Chauvier 1 , Lucie Finck 1 , Elisabeth Irran 1 , Martin Oestreich 1
Affiliation
|
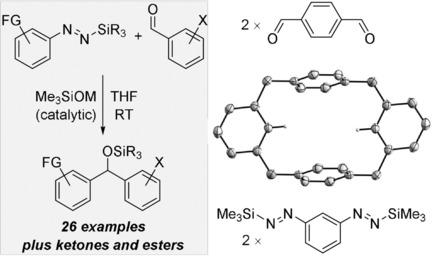
A method for the catalytic generation of functionalized aryl alkali metals is reported. These highly reactive intermediates are liberated from silyl‐protected aryl‐substituted diazenes by the action of Lewis basic alkali metal silanolates, resulting in desilylation and loss of N2. Catalytic quantities of these Lewis bases initiate the transfer of the aryl nucleophile from the diazene to carbonyl and carboxyl compounds with superb functional‐group tolerance. The aryl alkali metal can be decorated with electrophilic substituents such as methoxycarbonyl or cyano as well as halogen groups. The synthesis of a previously unknown cyclophane‐like [4]arene macrocycle from a 1,3‐bisdiazene combined with a 1,4‐dialdehyde underlines the potential of the approach.
中文翻译:

通过从 N-芳基-N'-甲硅烷基二氮烯中原位释放芳基亲核试剂来自催化羰基芳基化。
报道了一种催化生成官能化芳基碱金属的方法。这些高活性中间体通过路易斯碱性碱金属硅烷醇盐的作用从甲硅烷基保护的芳基取代的二氮烯中释放出来,导致脱甲硅烷基化和 N 2的损失。这些路易斯碱的催化量引发芳基亲核试剂从二氮烯转移到具有极好的官能团耐受性的羰基和羧基化合物。芳基碱金属可以用亲电取代基如甲氧基羰基或氰基以及卤素基团修饰。由 1,3-双二氮烯与 1,4-二醛结合合成以前未知的环芳类 [4] 芳烃大环,凸显了该方法的潜力。
更新日期:2020-02-06
中文翻译:

通过从 N-芳基-N'-甲硅烷基二氮烯中原位释放芳基亲核试剂来自催化羰基芳基化。
报道了一种催化生成官能化芳基碱金属的方法。这些高活性中间体通过路易斯碱性碱金属硅烷醇盐的作用从甲硅烷基保护的芳基取代的二氮烯中释放出来,导致脱甲硅烷基化和 N 2的损失。这些路易斯碱的催化量引发芳基亲核试剂从二氮烯转移到具有极好的官能团耐受性的羰基和羧基化合物。芳基碱金属可以用亲电取代基如甲氧基羰基或氰基以及卤素基团修饰。由 1,3-双二氮烯与 1,4-二醛结合合成以前未知的环芳类 [4] 芳烃大环,凸显了该方法的潜力。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号