当前位置:
X-MOL 学术
›
JACC Heart Fail.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Omecamtiv Mecarbil in Chronic Heart Failure With Reduced Ejection Fraction: Rationale and Design of GALACTIC-HF.
JACC: Heart Failure ( IF 10.3 ) Pub Date : 2020-02-06 , DOI: 10.1016/j.jchf.2019.12.001 John R Teerlink 1 , Rafael Diaz 2 , G Michael Felker 3 , John J V McMurray 4 , Marco Metra 5 , Scott D Solomon 6 , Jason C Legg 7 , Gustavo Büchele 7 , Claire Varin 8 , Christopher E Kurtz 7 , Fady I Malik 9 , Narimon Honarpour 7
JACC: Heart Failure ( IF 10.3 ) Pub Date : 2020-02-06 , DOI: 10.1016/j.jchf.2019.12.001 John R Teerlink 1 , Rafael Diaz 2 , G Michael Felker 3 , John J V McMurray 4 , Marco Metra 5 , Scott D Solomon 6 , Jason C Legg 7 , Gustavo Büchele 7 , Claire Varin 8 , Christopher E Kurtz 7 , Fady I Malik 9 , Narimon Honarpour 7
Affiliation
|
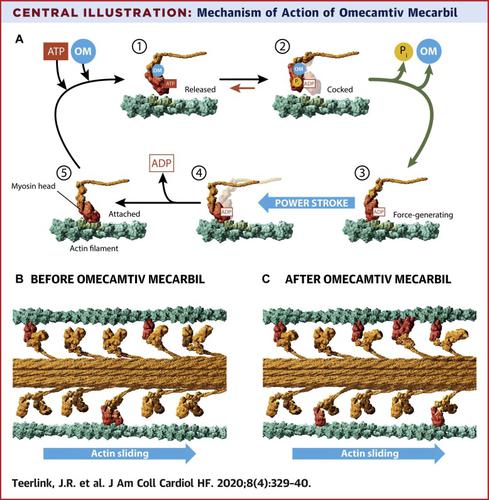
A central factor in the pathogenesis of heart failure (HF) with reduced ejection fraction is the initial decrease in systolic function. Prior attempts at increasing cardiac contractility with oral drugs have uniformly resulted in signals of increased mortality at pharmacologically effective doses. Omecamtiv mecarbil is a novel, selective cardiac myosin activator that has been shown to improve cardiac function and to decrease ventricular volumes, heart rate, and N-terminal pro-B-type natriuretic peptide in patients with chronic HF. The GALACTIC-HF (Global Approach to Lowering Adverse Cardiac outcomes Through Improving Contractility in Heart Failure) trial tests the hypotheses that omecamtiv mecarbil can safely improve symptoms, prevent clinical HF events, and delay CV death in patients with chronic HF. The GALACTIC-HF trial is an international, multicenter, randomized, double-blind, placebo-controlled, event-driven cardiovascular outcomes trial. More than 8,000 patients with chronic symptomatic (New York Heart Association functional class II to IV) HF, left ventricular ejection fraction ≤35%, elevated natriuretic peptides, and either current hospitalization for HF or history of hospitalization or emergency department visit for HF within a year of screening will be randomized to either oral placebo or omecamtiv mecarbil employing a pharmacokinetic-guided dose titration strategy using doses of 25, 37.5, or 50 mg twice daily. The primary efficacy outcome is the time to cardiovascular death or first HF event. The study has 90% power to assess a final hazard ratio of approximately 0.80 in cardiovascular death, the first secondary outcome. The GALACTIC-HF trial is the first trial examining whether selectively increasing cardiac contractility in patients with HF with reduced ejection fraction will result in improved clinical outcomes. (Registrational Study With Omecamtiv Mecarbil/AMG 423 to Treat Chronic Heart Failure With Reduced Ejection Fraction [GALACTIC-HF]; NCT02929329).
中文翻译:

慢性心力衰竭伴有射血分数降低的Omecamtiv Mecarbil:GALACTIC-HF的原理和设计。
射血分数降低的心力衰竭(HF)发病机理的中心因素是收缩功能的最初降低。先前尝试通过口服药物增加心脏收缩力的尝试统一地导致在药理有效剂量下死亡率增加的信号。Omecamtiv mecarbil是一种新型的选择性心肌肌球蛋白激活剂,已被证明可改善慢性HF患者的心脏功能,并降低其心室容量,心律和N端pro-B型利钠肽。GALACTIC-HF(通过改善心力衰竭的收缩力来降低不良心脏结局的全球方法)试验检验了以下假设:奥美替卡韦可安全地改善慢性HF患者的症状,预防临床HF事件并延迟CV死亡。GALACTIC-HF试验是一项国际试验,多中心,随机,双盲,安慰剂对照,事件驱动的心血管结果试验。8,000多名患有慢性症状(纽约心脏协会功能性II至IV级)的HF,左心室射血分数≤35%,利钠肽水平升高,既往因HF住院或住院或急诊就诊的HF患者一年的筛选将采用药代动力学指导的剂量滴定策略随机分为口服安慰剂或omecamtiv mecarbil,每天两次,剂量分别为25、37.5或50 mg。主要疗效结果是心血管死亡或首次HF事件发生的时间。这项研究有90%的能力可以评估心血管死亡(首个次要结局)的最终危险比约为0.80。GALACTIC-HF试验是第一项试验,该试验研究是否选择性提高射血分数降低的HF患者的心脏收缩能力是否会改善临床结果。(使用Omecamtiv Mecarbil / AMG 423进行登记研究以减少射血分数[GALACTIC-HF]治疗慢性心力衰竭; NCT02929329)。
更新日期:2020-02-06
中文翻译:

慢性心力衰竭伴有射血分数降低的Omecamtiv Mecarbil:GALACTIC-HF的原理和设计。
射血分数降低的心力衰竭(HF)发病机理的中心因素是收缩功能的最初降低。先前尝试通过口服药物增加心脏收缩力的尝试统一地导致在药理有效剂量下死亡率增加的信号。Omecamtiv mecarbil是一种新型的选择性心肌肌球蛋白激活剂,已被证明可改善慢性HF患者的心脏功能,并降低其心室容量,心律和N端pro-B型利钠肽。GALACTIC-HF(通过改善心力衰竭的收缩力来降低不良心脏结局的全球方法)试验检验了以下假设:奥美替卡韦可安全地改善慢性HF患者的症状,预防临床HF事件并延迟CV死亡。GALACTIC-HF试验是一项国际试验,多中心,随机,双盲,安慰剂对照,事件驱动的心血管结果试验。8,000多名患有慢性症状(纽约心脏协会功能性II至IV级)的HF,左心室射血分数≤35%,利钠肽水平升高,既往因HF住院或住院或急诊就诊的HF患者一年的筛选将采用药代动力学指导的剂量滴定策略随机分为口服安慰剂或omecamtiv mecarbil,每天两次,剂量分别为25、37.5或50 mg。主要疗效结果是心血管死亡或首次HF事件发生的时间。这项研究有90%的能力可以评估心血管死亡(首个次要结局)的最终危险比约为0.80。GALACTIC-HF试验是第一项试验,该试验研究是否选择性提高射血分数降低的HF患者的心脏收缩能力是否会改善临床结果。(使用Omecamtiv Mecarbil / AMG 423进行登记研究以减少射血分数[GALACTIC-HF]治疗慢性心力衰竭; NCT02929329)。






























 京公网安备 11010802027423号
京公网安备 11010802027423号