当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Oxidation of CO by N2O Enabled by Al2O2/3+: Temperature Dependent Kinetics and Statistical Modeling.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-02-19 , DOI: 10.1021/acs.jpca.9b10732 Brendan C Sweeny 1 , David C McDonald 1 , Jennifer L Poutsma 2 , John C Poutsma 3 , Nicholas S Shuman 1 , Shaun G Ard 1 , Albert A Viggiano 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-02-19 , DOI: 10.1021/acs.jpca.9b10732 Brendan C Sweeny 1 , David C McDonald 1 , Jennifer L Poutsma 2 , John C Poutsma 3 , Nicholas S Shuman 1 , Shaun G Ard 1 , Albert A Viggiano 1
Affiliation
|
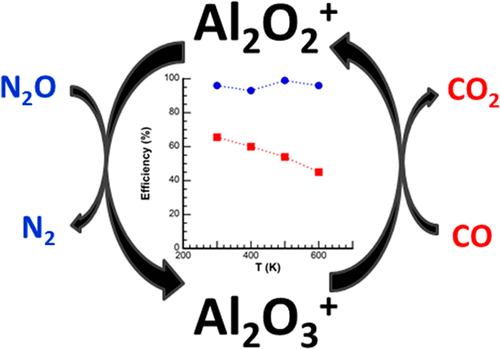
The reactions of Al2O2+ + N2O and Al2O3+ + CO, forming a catalytic cycle oxidizing CO by N2O, have been investigated from 300 to 600 K in a variable ion source, temperature adjustable, selected-ion flow tube (VISTA-SIFT). Reaction coordinates have been calculated using density functional theory and statistical modeling of those surfaces compared to experimental kinetics data for mechanistic insight. The reaction of Al2O2+ + N2O proceeds at the Su-Chesnavich collisional limit at all temperatures studied, yielding only Al2O3+, with the exception of a small (<5%) amount of association product, Al2O2(N2O)+ at 300 K. The reaction of Al2O3+ with CO produces Al2O2+ with a rate constant of 4.7 ± 1.2 × 10-10 cm3 s-1 at 300 K, decreasing with temperature as T-0.5±0.2. In addition, a significant amount of association product, Al2O3(CO)+, was observed with rate constants for formation ranging from 10-11 to 10-10 cm3 s-1 dependent upon He buffer gas concentration and temperature. Implications of these kinetic measurements with regard to the reactive surface are discussed.
中文翻译:

Al2O2 / 3 +活化的N2O催化CO氧化CO:温度依赖性动力学和统计模型。
已在300至600 K的可变离子源,温度可调的选择离子流量管(VISTA-SIFT)中研究了Al2O2 + + N2O和Al2O3 + + CO的反应,该反应通过N2O氧化氧化CO。反应密度是使用密度泛函理论和那些表面的统计模型与实验动力学数据进行对比计算得出的,从而可以了解机理。在研究的所有温度下,Al2O2 + + N2O的反应均在Su-Chesnavich碰撞极限下进行,仅产生Al2O3 +,只有少量(<5%)的缔合产物Al2O2(N2O)+在300 K下发生反应。在300 K下,用CO溶解Al2O3 +会生成速率常数为4.7±1.2×10-10 cm3 s-1的Al2O2 +,随温度的升高而降低,为T-0.5±0.2。此外,大量的缔合产物Al2O3(CO)+ 根据氦缓冲气体的浓度和温度,可以观察到生成速率常数为10-11至10-10 cm3 s-1。讨论了这些动力学测量对反应表面的影响。
更新日期:2020-02-20
中文翻译:

Al2O2 / 3 +活化的N2O催化CO氧化CO:温度依赖性动力学和统计模型。
已在300至600 K的可变离子源,温度可调的选择离子流量管(VISTA-SIFT)中研究了Al2O2 + + N2O和Al2O3 + + CO的反应,该反应通过N2O氧化氧化CO。反应密度是使用密度泛函理论和那些表面的统计模型与实验动力学数据进行对比计算得出的,从而可以了解机理。在研究的所有温度下,Al2O2 + + N2O的反应均在Su-Chesnavich碰撞极限下进行,仅产生Al2O3 +,只有少量(<5%)的缔合产物Al2O2(N2O)+在300 K下发生反应。在300 K下,用CO溶解Al2O3 +会生成速率常数为4.7±1.2×10-10 cm3 s-1的Al2O2 +,随温度的升高而降低,为T-0.5±0.2。此外,大量的缔合产物Al2O3(CO)+ 根据氦缓冲气体的浓度和温度,可以观察到生成速率常数为10-11至10-10 cm3 s-1。讨论了这些动力学测量对反应表面的影响。













































 京公网安备 11010802027423号
京公网安备 11010802027423号