当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multiple Mechanisms Mapped in Aryl Alkyl Ether Cleavage via Aqueous Electrocatalytic Hydrogenation (ECH) over Skeletal Nickel
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-02-04 , DOI: 10.1021/jacs.0c00199 Yuting Zhou , Grace E. Klinger , Eric L. Hegg , Christopher M. Saffron , James E. Jackson
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-02-04 , DOI: 10.1021/jacs.0c00199 Yuting Zhou , Grace E. Klinger , Eric L. Hegg , Christopher M. Saffron , James E. Jackson
|
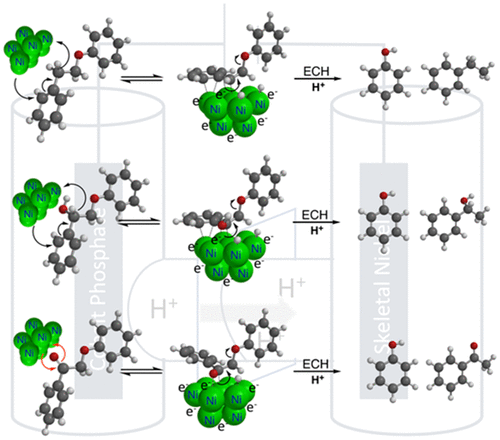
We present here detailed mechanistic studies of electrocatalytic hydrogenation (ECH) in aqueous solution over skeletal nickel cathodes to probe the various paths of reductive catalytic C-O bond cleavage among functionalized aryl ethers relevant to energy science. Heterogeneous catalytic hydrogenolysis of aryl ethers is important both in hydrodeoxygenation of fossil fuels and in upgrading of lignin from biomass. The presence or absence of simple functionalities such as carbonyl, hydroxyl, methyl or methoxyl groups is known to cause dramatic shifts in reactivity and cleavage selectivity between sp3 C-O and sp2 C-O bonds. Specifically, reported hydrogenolysis studies with Ni and other catalysts have hinted at different cleavage mechanisms for the C-O ether bonds in α-keto and α-hydroxy β-O-4 type aryl ether linkages of lignin. Our new rate, selectivity, and isotopic labeling results from ECH reactions confirm that these aryl ethers undergo C-O cleavage via distinct paths. For the simple 2-phenoxy-1-phenylethane or its alcohol congener, 2-phenoxy-1-phenylethanol, the benzylic site is activated via Ni C-H insertion, followed by beta elimination of the phenoxide leaving group. But in the case of the ketone, 2-phenoxyacetophenone, the polarized carbonyl π system apparently binds directly with the electron rich Ni cathode surface without breaking the aromaticity of the neighboring phenyl ring, leading to rapid cleavage. Substituent steric and electronic perturbations across a broad range of β-O-4 type ethers create a hierarchy of cleavage rates that supports these mechanistic ideas while offering guidance to al-low rational design of the catalytic method. On the basis of the new insights, the usage of co-solvent acetone is shown to enable control of product selectivity.
中文翻译:

通过骨架镍上的水性电催化氢化(ECH)在芳基烷基醚裂解中映射的多种机制
我们在此详细介绍了骨架镍阴极上水溶液中电催化加氢 (ECH) 的机理研究,以探索与能源科学相关的官能化芳基醚之间还原催化 CO 键裂解的各种途径。芳基醚的多相催化氢解在化石燃料的加氢脱氧和生物质木质素的升级中都很重要。已知简单官能团(如羰基、羟基、甲基或甲氧基)的存在或不存在会导致 sp3 CO 和 sp2 CO 键之间的反应性和裂解选择性发生显着变化。具体而言,已报道的 Ni 和其他催化剂的氢解研究暗示了木质素的 α-酮和 α-羟基 β-O-4 型芳基醚键中 CO 醚键的不同裂解机制。我们的新费率,ECH 反应的选择性和同位素标记结果证实这些芳基醚通过不同的路径进行 CO 裂解。对于简单的 2-苯氧基-1-苯基乙烷或其醇同系物 2-苯氧基-1-苯基乙醇,苄基位点通过 Ni CH 插入激活,然后β 消除苯氧基离去基团。但是在酮的情况下,2-苯氧基苯乙酮,极化的羰基 π 系统显然直接与富电子的 Ni 阴极表面结合,而不会破坏相邻苯环的芳香性,导致快速裂解。广泛的 β-O-4 型醚中的取代位位阻和电子扰动创造了一个裂解速率等级,支持这些机械思想,同时为允许合理设计催化方法提供指导。在新见解的基础上,
更新日期:2020-02-04
中文翻译:

通过骨架镍上的水性电催化氢化(ECH)在芳基烷基醚裂解中映射的多种机制
我们在此详细介绍了骨架镍阴极上水溶液中电催化加氢 (ECH) 的机理研究,以探索与能源科学相关的官能化芳基醚之间还原催化 CO 键裂解的各种途径。芳基醚的多相催化氢解在化石燃料的加氢脱氧和生物质木质素的升级中都很重要。已知简单官能团(如羰基、羟基、甲基或甲氧基)的存在或不存在会导致 sp3 CO 和 sp2 CO 键之间的反应性和裂解选择性发生显着变化。具体而言,已报道的 Ni 和其他催化剂的氢解研究暗示了木质素的 α-酮和 α-羟基 β-O-4 型芳基醚键中 CO 醚键的不同裂解机制。我们的新费率,ECH 反应的选择性和同位素标记结果证实这些芳基醚通过不同的路径进行 CO 裂解。对于简单的 2-苯氧基-1-苯基乙烷或其醇同系物 2-苯氧基-1-苯基乙醇,苄基位点通过 Ni CH 插入激活,然后β 消除苯氧基离去基团。但是在酮的情况下,2-苯氧基苯乙酮,极化的羰基 π 系统显然直接与富电子的 Ni 阴极表面结合,而不会破坏相邻苯环的芳香性,导致快速裂解。广泛的 β-O-4 型醚中的取代位位阻和电子扰动创造了一个裂解速率等级,支持这些机械思想,同时为允许合理设计催化方法提供指导。在新见解的基础上,





















































 京公网安备 11010802027423号
京公网安备 11010802027423号