当前位置:
X-MOL 学术
›
Metabolism
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
IDH1-dependent α-KG regulates brown fat differentiation and function by modulating histone methylation.
Metabolism ( IF 10.8 ) Pub Date : 2020-02-05 , DOI: 10.1016/j.metabol.2020.154173 Hyun Sup Kang 1 , Jae Ho Lee 2 , Kyoung-Jin Oh 1 , Eun Woo Lee 2 , Baek Soo Han 1 , Kun-Young Park 3 , Jae Myoung Suh 4 , Jeong-Ki Min 1 , Seung-Wook Chi 1 , Sang Chul Lee 1 , Kwang-Hee Bae 1 , Won Kon Kim 1
Metabolism ( IF 10.8 ) Pub Date : 2020-02-05 , DOI: 10.1016/j.metabol.2020.154173 Hyun Sup Kang 1 , Jae Ho Lee 2 , Kyoung-Jin Oh 1 , Eun Woo Lee 2 , Baek Soo Han 1 , Kun-Young Park 3 , Jae Myoung Suh 4 , Jeong-Ki Min 1 , Seung-Wook Chi 1 , Sang Chul Lee 1 , Kwang-Hee Bae 1 , Won Kon Kim 1
Affiliation
|
OBJECTIVE
Brown adipocytes play important roles in the regulation of energy homeostasis by uncoupling protein 1-mediated non-shivering thermogenesis. Recent studies suggest that brown adipocytes as novel therapeutic targets for combating obesity and associated diseases, such as type II diabetes. However, the molecular mechanisms underlying brown adipocyte differentiation and function are not fully understood.
METHODS
We employed previous findings obtained through proteomic studies performed to assess proteins displaying altered levels during brown adipocyte differentiation. Here, we performed assays to determine the functional significance of their altered levels during brown adipogenesis and development.
RESULTS
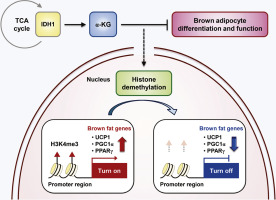
We identified isocitrate dehydrogenase 1 (IDH1) as upregulated during brown adipocyte differentiation, with subsequent investigations revealing that ectopic expression of IDH1 inhibited brown adipogenesis, whereas suppression of IDH1 levels promoted differentiation of brown adipocytes. Additionally, Idh1 overexpression resulted in increased levels of intracellular α-ketoglutarate (α-KG) and inhibited the expression of genes involved in brown adipogenesis. Exogenous treatment with α-KG reduced brown adipogenesis during the early phase of differentiation, and ChIP analysis revealed that IDH1-mediated α-KG reduced trimethylation of histone H3 lysine 4 in the promoters of genes associated with brown adipogenesis. Furthermore, administration of α-KG decreased adipogenic gene expression by modulating histone methylation in brown adipose tissues of mice.
CONCLUSION
These results suggested that the IDH1-α-KG axis plays an important role in regulating brown adipocyte differentiation and might represent a therapeutic target for treating metabolic diseases.
中文翻译:

依赖IDH1的α-KG通过调节组蛋白甲基化来调节棕色脂肪的分化和功能。
目的棕色脂肪细胞通过解偶联蛋白1介导的非颤动生热作用在能量稳态中发挥重要作用。最近的研究表明,褐色脂肪细胞是对抗肥胖症和相关疾病(如II型糖尿病)的新型治疗靶标。但是,尚未完全了解褐色脂肪细胞分化和功能的分子机制。方法我们采用通过蛋白质组学研究获得的先前发现,以评估在棕色脂肪细胞分化过程中蛋白质水平发生变化的蛋白质。在这里,我们进行了测定以确定它们在褐色脂肪形成和发育过程中改变水平的功能意义。结果我们鉴定出异柠檬酸脱氢酶1(IDH1)在褐色脂肪细胞分化过程中被上调,随后的研究表明,IDH1的异位表达抑制了褐色脂肪形成,而抑制IDH1的水平则促进了褐色脂肪细胞的分化。此外,Idh1过表达导致细胞内α-酮戊二酸(α-KG)水平升高,并抑制了参与褐色脂肪形成的基因的表达。α-KG的外源处理减少了分化早期的棕色脂肪形成,ChIP分析显示IDH1介导的α-KG减少了与棕色脂肪形成相关的基因启动子中组蛋白H3赖氨酸4的三甲基化。此外,通过调节小鼠棕色脂肪组织中的组蛋白甲基化,α-KG的施用降低了成脂基因的表达。
更新日期:2020-02-06
中文翻译:

依赖IDH1的α-KG通过调节组蛋白甲基化来调节棕色脂肪的分化和功能。
目的棕色脂肪细胞通过解偶联蛋白1介导的非颤动生热作用在能量稳态中发挥重要作用。最近的研究表明,褐色脂肪细胞是对抗肥胖症和相关疾病(如II型糖尿病)的新型治疗靶标。但是,尚未完全了解褐色脂肪细胞分化和功能的分子机制。方法我们采用通过蛋白质组学研究获得的先前发现,以评估在棕色脂肪细胞分化过程中蛋白质水平发生变化的蛋白质。在这里,我们进行了测定以确定它们在褐色脂肪形成和发育过程中改变水平的功能意义。结果我们鉴定出异柠檬酸脱氢酶1(IDH1)在褐色脂肪细胞分化过程中被上调,随后的研究表明,IDH1的异位表达抑制了褐色脂肪形成,而抑制IDH1的水平则促进了褐色脂肪细胞的分化。此外,Idh1过表达导致细胞内α-酮戊二酸(α-KG)水平升高,并抑制了参与褐色脂肪形成的基因的表达。α-KG的外源处理减少了分化早期的棕色脂肪形成,ChIP分析显示IDH1介导的α-KG减少了与棕色脂肪形成相关的基因启动子中组蛋白H3赖氨酸4的三甲基化。此外,通过调节小鼠棕色脂肪组织中的组蛋白甲基化,α-KG的施用降低了成脂基因的表达。































 京公网安备 11010802027423号
京公网安备 11010802027423号