Nature ( IF 50.5 ) Pub Date : 2020-02-05 , DOI: 10.1038/s41586-020-1995-4 Francesca Coscia 1 , Ajda Taler-Verčič 2, 3 , Veronica T Chang 1 , Ludwig Sinn 4 , Francis J O'Reilly 4 , Thierry Izoré 1 , Miha Renko 2 , Imre Berger 5 , Juri Rappsilber 4, 6 , Dušan Turk 2, 3 , Jan Löwe 1
|
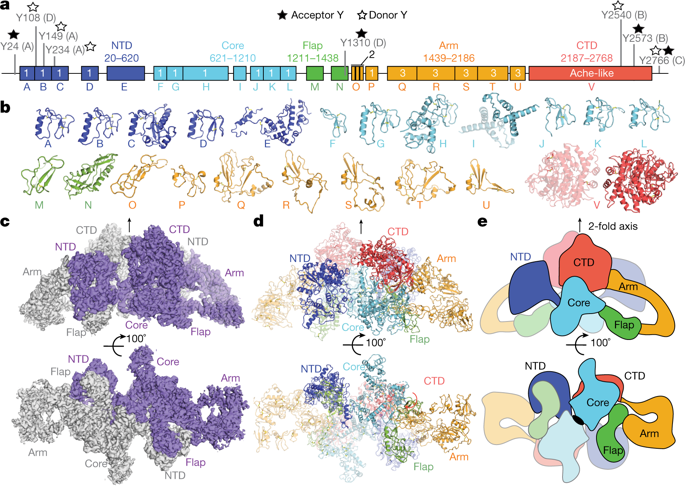
Thyroglobulin (TG) is the protein precursor of thyroid hormones, which are essential for growth, development and the control of metabolism in vertebrates1,2. Hormone synthesis from TG occurs in the thyroid gland via the iodination and coupling of pairs of tyrosines, and is completed by TG proteolysis3. Tyrosine proximity within TG is thought to enable the coupling reaction but hormonogenic tyrosines have not been clearly identified, and the lack of a three-dimensional structure of TG has prevented mechanistic understanding4. Here we present the structure of full-length human thyroglobulin at a resolution of approximately 3.5 Å, determined by cryo-electron microscopy. We identified all of the hormonogenic tyrosine pairs in the structure, and verified them using site-directed mutagenesis and in vitro hormone-production assays using human TG expressed in HEK293T cells. Our analysis revealed that the proximity, flexibility and solvent exposure of the tyrosines are the key characteristics of hormonogenic sites. We transferred the reaction sites from TG to an engineered tyrosine donor–acceptor pair in the unrelated bacterial maltose-binding protein (MBP), which yielded hormone production with an efficiency comparable to that of TG. Our study provides a framework to further understand the production and regulation of thyroid hormones.
中文翻译:

人甲状腺球蛋白的结构
甲状腺球蛋白 (TG) 是甲状腺激素的蛋白质前体,对于脊椎动物的生长、发育和代谢控制至关重要1,2 。 TG 的激素合成在甲状腺中通过酪氨酸对的碘化和偶联进行,并通过 TG 蛋白水解完成3 。 TG 内的酪氨酸接近被认为能够实现偶联反应,但激素酪氨酸尚未明确识别,并且 TG 三维结构的缺乏阻碍了机制理解4 。在这里,我们展示了通过冷冻电子显微镜测定的全长人类甲状腺球蛋白的结构,分辨率约为 3.5 Å。我们鉴定了该结构中的所有激素酪氨酸对,并使用 HEK293T 细胞中表达的人 TG 进行定点诱变和体外激素产生测定来验证它们。我们的分析表明,酪氨酸的邻近性、灵活性和溶剂暴露性是激素生成位点的关键特征。我们将反应位点从 TG 转移到不相关的细菌麦芽糖结合蛋白 (MBP) 中的工程酪氨酸供体-受体对,这产生了与 TG 相当的激素生产效率。我们的研究为进一步了解甲状腺激素的产生和调节提供了一个框架。













































 京公网安备 11010802027423号
京公网安备 11010802027423号