当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transport Mechanism of Acetamide in Deep Eutectic Solvents.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-02-17 , DOI: 10.1021/acs.jpcb.9b11137 H Srinivasan 1 , V K Sharma 1 , V García Sakai 2 , Jan P Embs 3 , R Mukhopadhyay 1, 4 , S Mitra 1, 4
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-02-17 , DOI: 10.1021/acs.jpcb.9b11137 H Srinivasan 1 , V K Sharma 1 , V García Sakai 2 , Jan P Embs 3 , R Mukhopadhyay 1, 4 , S Mitra 1, 4
Affiliation
|
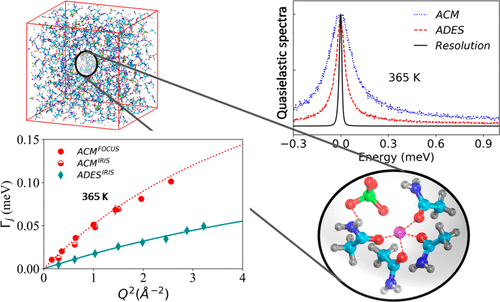
Over the last couple of decades, deep eutectic solvents (DESs) have emerged as novel alternatives to ionic liquids that are extensively used in the synthesis of innovative materials, metal processing, catalysis, etc. However, their usage is limited, primarily because of their large viscosity and poor conductivity. Therefore, an understanding of the molecular origin of these transport properties is essential to improve their industrial applicability. Here, we present the report of the nanoscopic diffusion mechanism of acetamide in a DES synthesized with lithium perchlorate as studied using neutron scattering and molecular dynamics (MD) simulation techniques. A diffusion model is constructed with the help of MD simulation data comprising two distinct processes, corresponding to long-range jump diffusion and localized diffusion within a restricted volume. This diffusion model is validated through the analysis of neutron scattering data in the acetamide based DES (ADES) and molten acetamide. Although ADES has a remarkably lower freezing point compared to pure acetamide, the molecular mobility is found to be enormously restricted in the former. Particularly, the long-range jump diffusion process of acetamide is slower by a factor of 3 in ADES in comparison with molten acetamide. Further, the geometry of localized diffusion is found to be unaltered, but the dynamics is observed to be slightly slower in ADES. The diffusion model is found to be consistent over a wide temperature range for the ADES. Both long-range and localized diffusion show Arrhenius dependence with temperature in ADES. MD simulation analysis reveals that the long-range diffusion in ADES is restricted mainly due to the formation of hydrogen bond mediated complexes between the ionic species of the salt and acetamide molecules. Hence, the origin of higher viscosity observed in ADES can be attributed to the complexation in the ADES. The complex formation also explains the inhibition of the crystallization process while cooling and thereby results in depression of the freezing point of ADES.
中文翻译:

乙酰胺在深共晶溶剂中的传输机理。
在过去的几十年中,深共晶溶剂(DES)逐渐成为离子液体的新型替代品,这些离子液体被广泛用于创新材料的合成,金属加工,催化等方面。然而,它们的使用受到限制,主要是因为它们粘度大,导电性差。因此,对这些传输特性的分子起源的理解对于提高它们的工业适用性至关重要。在这里,我们介绍了乙酰胺在高氯酸锂合成的DES中的纳米扩散机理的报告,这是使用中子散射和分子动力学(MD)模拟技术研究的。借助包含两个不同过程的MD仿真数据构建扩散模型,对应于有限体积内的远距离跳跃扩散和局部扩散。通过分析基于乙酰胺的DES(ADES)和熔融乙酰胺中的中子散射数据,验证了该扩散模型。尽管与纯乙酰胺相比,ADES的凝固点低很多,但发现前者的分子迁移受到极大限制。特别是,与熔融乙酰胺相比,ADES中乙酰胺的长距离跳跃扩散过程要慢3倍。此外,发现局部扩散的几何形状没有改变,但是在ADES中观察到动力学稍微慢一些。发现扩散模型在ADES的宽温度范围内是一致的。在ADES中,远距离扩散和局部扩散都显示出Arrhenius对温度的依赖性。MD模拟分析表明,ADES中的远程扩散受到限制,主要是由于盐和乙酰胺分子的离子种类之间氢键介导的配合物的形成。因此,在ADES中观察到的较高粘度的起因可归因于ADES中的络合。复合物的形成还解释了在冷却时抑制结晶过程,从而导致ADES的凝固点降低。
更新日期:2020-02-17
中文翻译:

乙酰胺在深共晶溶剂中的传输机理。
在过去的几十年中,深共晶溶剂(DES)逐渐成为离子液体的新型替代品,这些离子液体被广泛用于创新材料的合成,金属加工,催化等方面。然而,它们的使用受到限制,主要是因为它们粘度大,导电性差。因此,对这些传输特性的分子起源的理解对于提高它们的工业适用性至关重要。在这里,我们介绍了乙酰胺在高氯酸锂合成的DES中的纳米扩散机理的报告,这是使用中子散射和分子动力学(MD)模拟技术研究的。借助包含两个不同过程的MD仿真数据构建扩散模型,对应于有限体积内的远距离跳跃扩散和局部扩散。通过分析基于乙酰胺的DES(ADES)和熔融乙酰胺中的中子散射数据,验证了该扩散模型。尽管与纯乙酰胺相比,ADES的凝固点低很多,但发现前者的分子迁移受到极大限制。特别是,与熔融乙酰胺相比,ADES中乙酰胺的长距离跳跃扩散过程要慢3倍。此外,发现局部扩散的几何形状没有改变,但是在ADES中观察到动力学稍微慢一些。发现扩散模型在ADES的宽温度范围内是一致的。在ADES中,远距离扩散和局部扩散都显示出Arrhenius对温度的依赖性。MD模拟分析表明,ADES中的远程扩散受到限制,主要是由于盐和乙酰胺分子的离子种类之间氢键介导的配合物的形成。因此,在ADES中观察到的较高粘度的起因可归因于ADES中的络合。复合物的形成还解释了在冷却时抑制结晶过程,从而导致ADES的凝固点降低。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号