当前位置:
X-MOL 学术
›
Cell Death Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
TfR1 binding with H-ferritin nanocarrier achieves prognostic diagnosis and enhances the therapeutic efficacy in clinical gastric cancer.
Cell Death & Disease ( IF 8.1 ) Pub Date : 2020-02-05 , DOI: 10.1038/s41419-020-2272-z
Xiaojing Cheng 1 , Kelong Fan 2 , Lin Wang 1, 3, 4 , Xiangji Ying 3 , Andrew J Sanders 5 , Ting Guo 1 , Xiaofang Xing 1 , Meng Zhou 2 , Hong Du 1 , Ying Hu 6 , Huirong Ding 7 , Ziyu Li 3 , Xianzi Wen 1 , Wenguo Jiang 5 , Xiyun Yan 2 , Jiafu Ji 1, 3
Cell Death & Disease ( IF 8.1 ) Pub Date : 2020-02-05 , DOI: 10.1038/s41419-020-2272-z
Xiaojing Cheng 1 , Kelong Fan 2 , Lin Wang 1, 3, 4 , Xiangji Ying 3 , Andrew J Sanders 5 , Ting Guo 1 , Xiaofang Xing 1 , Meng Zhou 2 , Hong Du 1 , Ying Hu 6 , Huirong Ding 7 , Ziyu Li 3 , Xianzi Wen 1 , Wenguo Jiang 5 , Xiyun Yan 2 , Jiafu Ji 1, 3
Affiliation
|
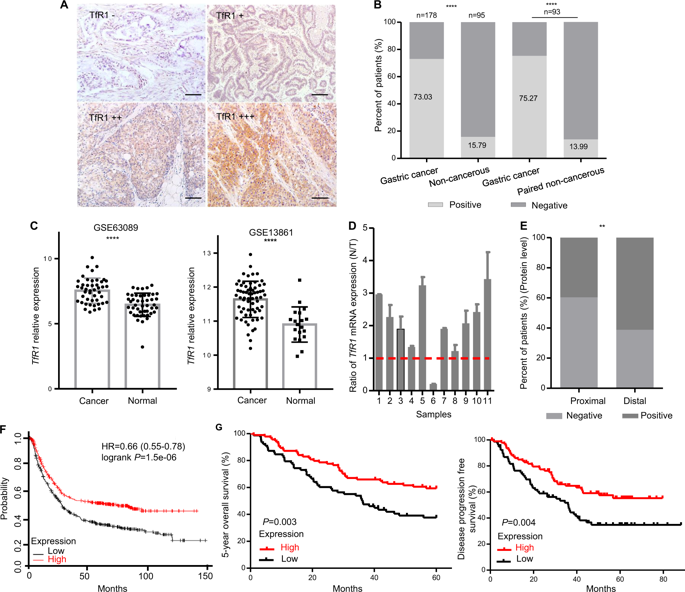
H-ferritin (HFn) nanocarrier is emerging as a promising theranostic platform for tumor diagnosis and therapy, which can specifically target tumor cells via binding transferrin receptor 1 (TfR1). This led us to investigate the therapeutic function of TfR1 in GC. The clinical significance of TfR1 was assessed in 178 GC tissues by using a magneto-HFn nanoparticle-based immunohistochemistry method. The therapeutic effects of doxorubicin-loaded HFn nanocarriers (HFn-Dox) were evaluated on TfR1-positive GC patient-derived xenograft (GC-PDX) models. The biological function of TfR1 was investigated through in vitro and in vivo assays. TfR1 was upregulated (73.03%) in GC tissues, and reversely correlated with patient outcome. TfR1-negative sorted cells exhibited tumor-initiating features, which enhanced tumor formation and migration/invasion, whereas TfR1-positive sorted cells showed significant proliferation ability. Knockout of TfR1 in GC cells also enhanced cell invasion. TfR1-deficient cells displayed immune escape by upregulating PD-L1, CXCL9, and CXCL10, when disposed with IFN-γ. Western blot results demonstrated that TfR1-knockout GC cells upregulated Akt and STAT3 signaling. Moreover, in TfR1-positive GC-PDX models, the HFn-Dox group significantly inhibited tumor growth, and increased mouse survival, compared with that of free-Dox group. TfR1 could be a potential prognostic and therapeutic biomarker for GC: (i) TfR1 reversely correlated with patient outcome, and its negative cells possessed tumor-aggressive features; (ii) TfR1-positive cells can be killed by HFn drug nanocarrier. Given the heterogeneity of GC, HFn drug nanocarrier combined with other therapies toward TfR1-negative cells (such as small molecules or immunotherapy) will be a new option for GC treatment.
中文翻译:

TfR1与H-铁蛋白纳米载体结合可实现临床胃癌的预后诊断并提高治疗效果。
H-铁蛋白 (HFn) 纳米载体正在成为一种有前景的肿瘤诊断和治疗治疗诊断平台,它可以通过结合转铁蛋白受体 1 (TfR1) 特异性靶向肿瘤细胞。这促使我们研究 TfR1 在 GC 中的治疗功能。使用基于磁性 HFn 纳米粒子的免疫组织化学方法评估了 178 个 GC 组织中 TfR1 的临床意义。在 TfR1 阳性 GC 患者来源的异种移植 (GC-PDX) 模型上评估了负载阿霉素的 HFn 纳米载体 (HFn-Dox) 的治疗效果。通过体外和体内测定研究了 TfR1 的生物学功能。 TfR1 在 GC 组织中表达上调 (73.03%),并且与患者预后呈负相关。 TfR1阴性分选细胞表现出肿瘤起始特征,增强肿瘤形成和迁移/侵袭,而TfR1阳性分选细胞则表现出显着的增殖能力。 GC 细胞中 TfR1 的敲除也增强了细胞侵袭。当用 IFN-γ 处理时,TfR1 缺陷细胞通过上调 PD-L1、CXCL9 和 CXCL10 表现出免疫逃逸。 Western blot 结果表明,TfR1 敲除的 GC 细胞上调 Akt 和 STAT3 信号传导。此外,在TfR1阳性GC-PDX模型中,与游离Dox组相比,HFn-Dox组显着抑制肿瘤生长,并提高小鼠存活率。 TfR1可能是GC的潜在预后和治疗生物标志物:(i)TfR1与患者预后呈负相关,其阴性细胞具有肿瘤侵袭性特征; (ii) HFn药物纳米载体可以杀死TfR1阳性细胞。鉴于GC的异质性,HFn药物纳米载体联合其他针对TfR1阴性细胞的疗法(如小分子或免疫疗法)将成为GC治疗的新选择。
更新日期:2020-02-06
中文翻译:

TfR1与H-铁蛋白纳米载体结合可实现临床胃癌的预后诊断并提高治疗效果。
H-铁蛋白 (HFn) 纳米载体正在成为一种有前景的肿瘤诊断和治疗治疗诊断平台,它可以通过结合转铁蛋白受体 1 (TfR1) 特异性靶向肿瘤细胞。这促使我们研究 TfR1 在 GC 中的治疗功能。使用基于磁性 HFn 纳米粒子的免疫组织化学方法评估了 178 个 GC 组织中 TfR1 的临床意义。在 TfR1 阳性 GC 患者来源的异种移植 (GC-PDX) 模型上评估了负载阿霉素的 HFn 纳米载体 (HFn-Dox) 的治疗效果。通过体外和体内测定研究了 TfR1 的生物学功能。 TfR1 在 GC 组织中表达上调 (73.03%),并且与患者预后呈负相关。 TfR1阴性分选细胞表现出肿瘤起始特征,增强肿瘤形成和迁移/侵袭,而TfR1阳性分选细胞则表现出显着的增殖能力。 GC 细胞中 TfR1 的敲除也增强了细胞侵袭。当用 IFN-γ 处理时,TfR1 缺陷细胞通过上调 PD-L1、CXCL9 和 CXCL10 表现出免疫逃逸。 Western blot 结果表明,TfR1 敲除的 GC 细胞上调 Akt 和 STAT3 信号传导。此外,在TfR1阳性GC-PDX模型中,与游离Dox组相比,HFn-Dox组显着抑制肿瘤生长,并提高小鼠存活率。 TfR1可能是GC的潜在预后和治疗生物标志物:(i)TfR1与患者预后呈负相关,其阴性细胞具有肿瘤侵袭性特征; (ii) HFn药物纳米载体可以杀死TfR1阳性细胞。鉴于GC的异质性,HFn药物纳米载体联合其他针对TfR1阴性细胞的疗法(如小分子或免疫疗法)将成为GC治疗的新选择。

































 京公网安备 11010802027423号
京公网安备 11010802027423号