当前位置:
X-MOL 学术
›
CNS Neurol. Disord. Drug Targets
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pharmacokinetics and Acute Toxicity of a Histone Deacetylase Inhibitor, Scriptaid, and its Neuroprotective Effects in Mice After Intracranial Hemorrhage.
CNS & Neurological Disorders - Drug Targets ( IF 2.7 ) Pub Date : 2020-01-31 , DOI: 10.2174/1871527319666191220111126 Heng Yang 1 , Xinjie Gao 1 , Jiabin Su 1 , Hanqiang Jiang 1 , Yu Lei 1 , Wei Ni 2 , Yuxiang Gu 2
中文翻译:

组蛋白去乙酰化酶抑制剂Scriptaid的药代动力学和急性毒性及其对颅内出血后小鼠的神经保护作用。
更新日期:2020-01-31
CNS & Neurological Disorders - Drug Targets ( IF 2.7 ) Pub Date : 2020-01-31 , DOI: 10.2174/1871527319666191220111126 Heng Yang 1 , Xinjie Gao 1 , Jiabin Su 1 , Hanqiang Jiang 1 , Yu Lei 1 , Wei Ni 2 , Yuxiang Gu 2
Affiliation
|
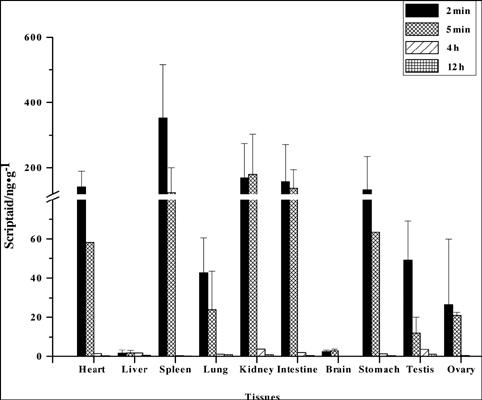
Background & Objective: The pharmacokinetics and acute toxicity of a histone deacetylase inhibitor, Scriptaid, was unknown in the mouse. The aim of this study was to determine the pharmacokinetics, acute toxicity, and tissue distribution of Scriptaid, a new histone deacetylase inhibitor, in mice, and its neuroprotective efficacy in a mouse intracranial hemorrhage (ICH) model.
Methods: The pharmacokinetics, acute toxicity, and tissue distribution were determined in C57BL/6 male and female mice after the intraperitoneal administration of a single dose. Behavioral tests, as well as investigations of brain atrophy and white matter injury, were used to evaluate the neuroprotective effect of Scriptaid after ICH. Western blotting was used to investigate if Scriptaid could offer antiinflammatory benefits after ICH. Results: No significant differences were observed in body weight or brain histopathology between the group that received Scriptaid at 50 mg/kg and the group that received dimethyl sulfoxide (control). The pharmacokinetics of Scriptaid in mice was nonlinear, and it was cleared rapidly at low doses and slowly at higher doses. Consistent with the pharmacokinetic data, Scriptaid was found to distribute in several tissues, including the spleen and kidneys. In the ICH model, we found that Scriptaid could reduce neurological deficits, brain atrophy, and white matter injury in a dose-dependent manner. Western blotting results demonstrated that Scriptaid could decrease the expression of pro-inflammatory cytokines IL1β and TNFα, as well as iNOS, after ICH. Conclusion: These findings indicate that Scriptaid is safe and can alleviate brain injury after ICH, thereby providing a foundation for the pharmacological action of Scriptaid in the treatment of brain injury after ICH.中文翻译:

组蛋白去乙酰化酶抑制剂Scriptaid的药代动力学和急性毒性及其对颅内出血后小鼠的神经保护作用。
背景与目的:在小鼠中尚不知道组蛋白脱乙酰基酶抑制剂Scriptaid的药代动力学和急性毒性。这项研究的目的是确定新型组蛋白脱乙酰基酶抑制剂Scriptaid在小鼠中的药代动力学,急性毒性和组织分布,及其在小鼠颅内出血(ICH)模型中的神经保护功效。
方法:腹膜内给药单剂量后,测定C57BL / 6雄性和雌性小鼠的药代动力学,急性毒性和组织分布。行为测试以及脑萎缩和白质损伤的调查被用于评估ICH后Scriptaid的神经保护作用。Western blotting用于研究Scriptaid在ICH后是否可以提供抗炎作用。结果:接受Scriptaid 50 mg / kg的组与接受二甲亚砜的组(对照组)在体重或脑组织病理学上未见明显差异。Scriptaid在小鼠体内的药代动力学是非线性的,在低剂量时迅速清除,在高剂量时缓慢清除。与药代动力学数据一致,发现Scriptaid分布在几个组织中,包括脾脏和肾脏。在ICH模型中,我们发现Scriptaid可以剂量依赖的方式减少神经功能缺损,脑萎缩和白质损伤。Western印迹结果表明,Scriptaid可以降低ICH后促炎细胞因子IL1β和TNFα以及iNOS的表达。 结论:这些发现表明,Scriptaid是安全的并且可以减轻ICH后的脑损伤,从而为Scriptaid在ICH后的脑损伤治疗中的药理作用奠定了基础。






























 京公网安备 11010802027423号
京公网安备 11010802027423号