Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cyclosporine H Improves the Multi-Vector Lentiviral Transduction of Murine Haematopoietic Progenitors and Stem Cells.
Scientific Reports ( IF 3.8 ) Pub Date : 2020-02-04 , DOI: 10.1038/s41598-020-58724-x Leonid Olender 1 , Nir Bujanover 1 , Omri Sharabi 1 , Oron Goldstein 1 , Roi Gazit 1
Scientific Reports ( IF 3.8 ) Pub Date : 2020-02-04 , DOI: 10.1038/s41598-020-58724-x Leonid Olender 1 , Nir Bujanover 1 , Omri Sharabi 1 , Oron Goldstein 1 , Roi Gazit 1
Affiliation
|
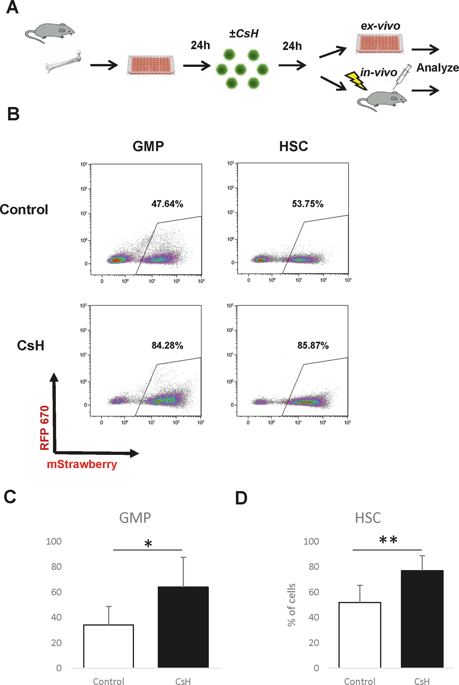
Haematopoietic stem cells (HSCs) have the potential for lifetime production of blood and immune cells. The introduction of transgenes into HSCs is important for basic research, as well as for multiple clinical applications, because HSC transplantation is an already established procedure. Recently, a major advancement has been reported in the use of cyclosporine H (CsH), which can significantly enhance the lentivirus (LV) transduction of human haematopoietic stem and progenitor cells (HSPCs). In this study, we employed CsH for LV transduction of murine HSCs and defined haematopoietic progenitors, confirming previous findings in more specific subsets of primitive haematopoietic cells. Our data confirm increased efficiencies, in agreement with the published data. We further experimented with the transduction with the simultaneous use of several vectors. The use of CsH yielded an even more robust increase in rates of multi-vector infection than the increase for a single-vector. CsH was reported to reduce the innate resistance mechanism against LV infection. We indeed found that additional pretreatment could increase the efficiency of transduction, in agreement with the originally reported results. Our data also suggest that CsH does not reduce the efficiency of transplantation into immune-competent hosts or the differentiation of HSCs while enhancing stable long-term expression in vivo. This new additive will surely help many studies in animal models and might be very useful for the development of novel HSC gene therapy approaches.
中文翻译:

环孢霉素H改善了小鼠造血祖细胞和干细胞的多载体慢病毒转导。
造血干细胞(HSC)具有终生生产血液和免疫细胞的潜力。将转基因引入HSC对于基础研究以及多种临床应用都非常重要,因为HSC移植已是既定程序。最近,已经报道了使用环孢霉素H(CsH)的重大进展,它可以显着增强人类造血干细胞和祖细胞(HSPC)的慢病毒(LV)转导。在这项研究中,我们将CsH用于小鼠HSC的LV转导并定义了造血祖细胞,从而证实了在原始造血细胞更特定子集中的先前发现。我们的数据证实了提高的效率,与已发布的数据一致。我们在同时使用几种载体的情况下进一步进行了转导实验。与单载体感染相比,CsH的使用在多载体感染率方面的提高甚至更为强劲。据报道,CsH可降低针对LV感染的先天性耐药机制。我们确实发现,额外的预处理可以提高转导效率,与最初报道的结果一致。我们的数据还表明,CsH不会降低移植到具有免疫能力的宿主的效率或HSC的分化,同时会增强体内稳定的长期表达。这种新的添加剂必将有助于动物模型的许多研究,并且可能对开发新型HSC基因治疗方法非常有用。与单载体感染相比,CsH的使用在多载体感染率方面的提高甚至更为强劲。据报道,CsH可降低针对LV感染的先天性耐药机制。我们确实发现,额外的预处理可以提高转导效率,与最初报道的结果一致。我们的数据还表明,CsH不会降低移植到具有免疫能力的宿主的效率或HSC的分化,同时会增强体内稳定的长期表达。这种新的添加剂必将有助于动物模型的许多研究,并且可能对开发新型HSC基因治疗方法非常有用。与单载体感染相比,CsH的使用导致了多载体感染率的提高甚至更为强劲。据报道,CsH可降低针对LV感染的先天性耐药机制。我们确实发现,额外的预处理可以提高转导效率,与最初报道的结果一致。我们的数据还表明,CsH不会降低移植到具有免疫能力的宿主的效率或HSC的分化,同时会增强体内稳定的长期表达。这种新的添加剂肯定会帮助许多动物模型研究,并且可能对开发新型HSC基因治疗方法非常有用。我们确实发现,额外的预处理可以提高转导效率,与最初报道的结果一致。我们的数据还表明,CsH不会降低移植到具有免疫能力的宿主的效率或HSC的分化,同时会增强体内稳定的长期表达。这种新的添加剂必将有助于动物模型的许多研究,并且可能对开发新型HSC基因治疗方法非常有用。我们确实发现,额外的预处理可以提高转导效率,与最初报道的结果一致。我们的数据还表明,CsH在增强体内稳定的长期表达的同时不会降低移植至具有免疫能力的宿主的效率或HSC的分化。这种新的添加剂必将有助于动物模型的许多研究,并且可能对开发新型HSC基因治疗方法非常有用。
更新日期:2020-02-04
中文翻译:

环孢霉素H改善了小鼠造血祖细胞和干细胞的多载体慢病毒转导。
造血干细胞(HSC)具有终生生产血液和免疫细胞的潜力。将转基因引入HSC对于基础研究以及多种临床应用都非常重要,因为HSC移植已是既定程序。最近,已经报道了使用环孢霉素H(CsH)的重大进展,它可以显着增强人类造血干细胞和祖细胞(HSPC)的慢病毒(LV)转导。在这项研究中,我们将CsH用于小鼠HSC的LV转导并定义了造血祖细胞,从而证实了在原始造血细胞更特定子集中的先前发现。我们的数据证实了提高的效率,与已发布的数据一致。我们在同时使用几种载体的情况下进一步进行了转导实验。与单载体感染相比,CsH的使用在多载体感染率方面的提高甚至更为强劲。据报道,CsH可降低针对LV感染的先天性耐药机制。我们确实发现,额外的预处理可以提高转导效率,与最初报道的结果一致。我们的数据还表明,CsH不会降低移植到具有免疫能力的宿主的效率或HSC的分化,同时会增强体内稳定的长期表达。这种新的添加剂必将有助于动物模型的许多研究,并且可能对开发新型HSC基因治疗方法非常有用。与单载体感染相比,CsH的使用在多载体感染率方面的提高甚至更为强劲。据报道,CsH可降低针对LV感染的先天性耐药机制。我们确实发现,额外的预处理可以提高转导效率,与最初报道的结果一致。我们的数据还表明,CsH不会降低移植到具有免疫能力的宿主的效率或HSC的分化,同时会增强体内稳定的长期表达。这种新的添加剂必将有助于动物模型的许多研究,并且可能对开发新型HSC基因治疗方法非常有用。与单载体感染相比,CsH的使用导致了多载体感染率的提高甚至更为强劲。据报道,CsH可降低针对LV感染的先天性耐药机制。我们确实发现,额外的预处理可以提高转导效率,与最初报道的结果一致。我们的数据还表明,CsH不会降低移植到具有免疫能力的宿主的效率或HSC的分化,同时会增强体内稳定的长期表达。这种新的添加剂肯定会帮助许多动物模型研究,并且可能对开发新型HSC基因治疗方法非常有用。我们确实发现,额外的预处理可以提高转导效率,与最初报道的结果一致。我们的数据还表明,CsH不会降低移植到具有免疫能力的宿主的效率或HSC的分化,同时会增强体内稳定的长期表达。这种新的添加剂必将有助于动物模型的许多研究,并且可能对开发新型HSC基因治疗方法非常有用。我们确实发现,额外的预处理可以提高转导效率,与最初报道的结果一致。我们的数据还表明,CsH在增强体内稳定的长期表达的同时不会降低移植至具有免疫能力的宿主的效率或HSC的分化。这种新的添加剂必将有助于动物模型的许多研究,并且可能对开发新型HSC基因治疗方法非常有用。































 京公网安备 11010802027423号
京公网安备 11010802027423号