当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Management of Hsp90-Dependent Protein Folding by Small Molecules Targeting the Aha1 Co-Chaperone.
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2020-02-03 , DOI: 10.1016/j.chembiol.2020.01.008 Jay K Singh 1 , Darren M Hutt 1 , Bradley Tait 2 , Naihsuan C Guy 3 , Jeffrey C Sivils 3 , Nina R Ortiz 3 , Ashley N Payan 3 , Shravan Kumar Komaragiri 4 , Jazzmin Jovonna Owens 4 , David Culbertson 5 , Laura J Blair 6 , Chad Dickey 6 , Szu Yu Kuo 7 , Dan Finley 8 , H Jane Dyson 5 , Marc B Cox 3 , Jaideep Chaudhary 4 , Jason E Gestwicki 7 , William E Balch 1
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2020-02-03 , DOI: 10.1016/j.chembiol.2020.01.008 Jay K Singh 1 , Darren M Hutt 1 , Bradley Tait 2 , Naihsuan C Guy 3 , Jeffrey C Sivils 3 , Nina R Ortiz 3 , Ashley N Payan 3 , Shravan Kumar Komaragiri 4 , Jazzmin Jovonna Owens 4 , David Culbertson 5 , Laura J Blair 6 , Chad Dickey 6 , Szu Yu Kuo 7 , Dan Finley 8 , H Jane Dyson 5 , Marc B Cox 3 , Jaideep Chaudhary 4 , Jason E Gestwicki 7 , William E Balch 1
Affiliation
|
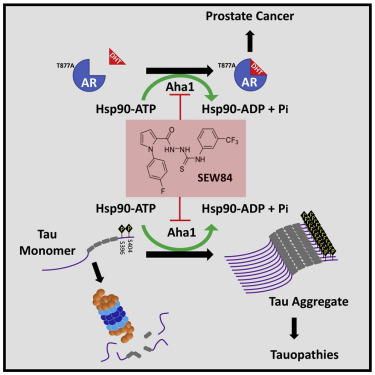
Hsp90 plays an important role in health and is a therapeutic target for managing misfolding disease. Compounds that disrupt co-chaperone delivery of clients to Hsp90 target a subset of Hsp90 activities, thereby minimizing the toxicity of pan-Hsp90 inhibitors. Here, we have identified SEW04784 as a first-in-class inhibitor of the Aha1-stimulated Hsp90 ATPase activity without inhibiting basal Hsp90 ATPase. Nuclear magnetic resonance analysis reveals that SEW84 binds to the C-terminal domain of Aha1 to weaken its asymmetric binding to Hsp90. Consistent with this observation, SEW84 blocks Aha1-dependent Hsp90 chaperoning activities, including the in vitro and in vivo refolding of firefly luciferase, and the transcriptional activity of the androgen receptor in cell-based models of prostate cancer and promotes the clearance of phosphorylated tau in cellular and tissue models of neurodegenerative tauopathy. We propose that SEW84 provides a novel lead scaffold for developing therapeutic approaches to treat proteostatic disease.
中文翻译:

通过针对 Aha1 辅助伴侣的小分子管理 Hsp90 依赖性蛋白质折叠。
Hsp90 在健康中发挥着重要作用,是控制错误折叠疾病的治疗靶点。破坏客户对 Hsp90 的共伴侣递送的化合物靶向 Hsp90 活性的子集,从而最大限度地减少泛 Hsp90 抑制剂的毒性。在这里,我们确定 SEW04784 是 Aha1 刺激的 Hsp90 ATP 酶活性的一流抑制剂,而不抑制基础 Hsp90 ATP 酶。核磁共振分析表明,SEW84 与 Aha1 的 C 端结构域结合,削弱了其与 Hsp90 的不对称结合。与这一观察结果一致,SEW84 阻断 Aha1 依赖性 Hsp90 陪伴活性,包括萤火虫荧光素酶的体外和体内重折叠,以及前列腺癌细胞模型中雄激素受体的转录活性,并促进磷酸化 tau 蛋白的清除。神经退行性 tau 病的细胞和组织模型。我们建议 SEW84 为开发治疗蛋白质疾病的治疗方法提供了一种新的先导支架。
更新日期:2020-02-04
中文翻译:

通过针对 Aha1 辅助伴侣的小分子管理 Hsp90 依赖性蛋白质折叠。
Hsp90 在健康中发挥着重要作用,是控制错误折叠疾病的治疗靶点。破坏客户对 Hsp90 的共伴侣递送的化合物靶向 Hsp90 活性的子集,从而最大限度地减少泛 Hsp90 抑制剂的毒性。在这里,我们确定 SEW04784 是 Aha1 刺激的 Hsp90 ATP 酶活性的一流抑制剂,而不抑制基础 Hsp90 ATP 酶。核磁共振分析表明,SEW84 与 Aha1 的 C 端结构域结合,削弱了其与 Hsp90 的不对称结合。与这一观察结果一致,SEW84 阻断 Aha1 依赖性 Hsp90 陪伴活性,包括萤火虫荧光素酶的体外和体内重折叠,以及前列腺癌细胞模型中雄激素受体的转录活性,并促进磷酸化 tau 蛋白的清除。神经退行性 tau 病的细胞和组织模型。我们建议 SEW84 为开发治疗蛋白质疾病的治疗方法提供了一种新的先导支架。















































 京公网安备 11010802027423号
京公网安备 11010802027423号