当前位置:
X-MOL 学术
›
Oncogenesis
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
SCFβ-TrCP-mediated degradation of TOP2β promotes cancer cell survival in response to chemotherapeutic drugs targeting topoisomerase II.
Oncogenesis ( IF 5.9 ) Pub Date : 2020-02-03 , DOI: 10.1038/s41389-020-0196-1 Jianfeng Shu 1, 2 , Danrui Cui 1, 2 , Ying Ma 1, 2 , Xiufang Xiong 2, 3 , Yi Sun 2, 3 , Yongchao Zhao 1, 2
Oncogenesis ( IF 5.9 ) Pub Date : 2020-02-03 , DOI: 10.1038/s41389-020-0196-1 Jianfeng Shu 1, 2 , Danrui Cui 1, 2 , Ying Ma 1, 2 , Xiufang Xiong 2, 3 , Yi Sun 2, 3 , Yongchao Zhao 1, 2
Affiliation
|
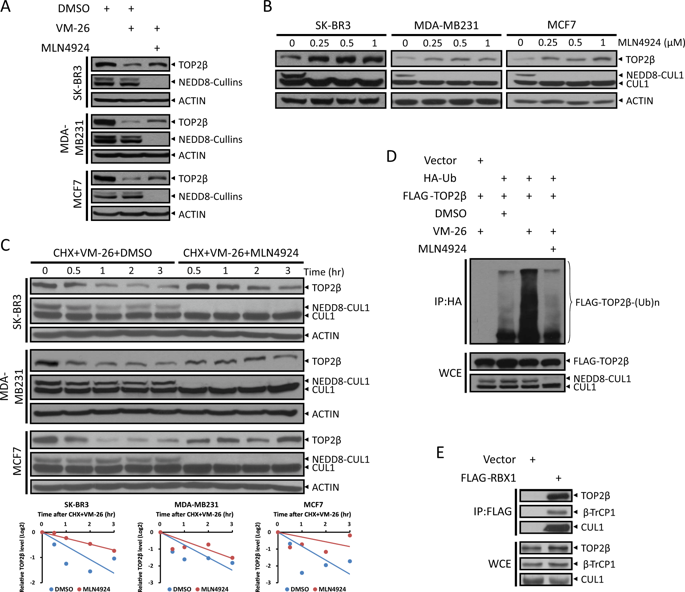
Topoisomerase II (TOP2)-targeting anticancer chemotherapeutic drugs, termed TOP2 poisons, are widely used and effective in the clinic by stabilizing TOP2-DNA covalent complexes to induce DNA double-strand breaks (DSBs) and ultimately, cause cell death. The stabilized TOP2-DNA complex is known to be degraded by proteasome, whereas the underlying mechanism for instant TOP2β degradation in response to TOP2 poisons and the subsequent biological consequence remain elusive. Here, we reported that TOP2 poison-induced TOP2β degradation is mediated by SCFβ-TrCP ubiquitin ligase. Specifically, DNA damage signal, triggered by teniposide (VM-26) treatment, activates ATM, cooperating with CK1 to phosphorylate TOP2β on Ser1134 and Ser1130, respectively, in a canonical degron motif to facilitate β-TrCP binding and subsequent degradation. Inactivation of ATM, CK1 or SCFβ-TrCP by small molecular inhibitors or genetic knockdown/knockout abrogates TOP2β degradation. Biologically, blockage of TOP2β degradation in combination with VM-26 treatment impairs DNA damage response and repair, leading to an accelerated cell death via apoptosis. Thus, it appears that TOP2β degradation is a cellular defensive mechanism to facilitate the exposure of DSBs to trigger DNA damage response and repair. Collectively, our findings reveal a new strategy to improve the efficacy of TOP2 poisons in combination with small-molecule inhibitors against TOP2β degradation.
中文翻译:

SCFβ-TrCP介导的TOP2β降解可响应靶向拓扑异构酶II的化学治疗药物促进癌细胞存活。
靶向拓扑异构酶II(TOP2)的抗癌化学治疗药物,称为TOP2毒物,通过稳定TOP2-DNA共价复合物以诱导DNA双链断裂(DSB)并最终导致细胞死亡,在临床上得到广泛使用和有效。已知稳定的TOP2-DNA复合物可被蛋白酶体降解,而响应TOP2毒物而导致的即时TOP2β降解的基本机制以及随后的生物学后果仍然难以捉摸。在这里,我们报道了TOP2毒物诱导的TOP2β降解是由SCFβ-TrCP泛素连接酶介导的。具体而言,由替尼泊苷(VM-26)处理触发的DNA损伤信号激活ATM,并与CK1协同作用以规范化的德格伦基序磷酸化Ser1134和Ser1130上的TOP2β,以促进β-TrCP结合和随后的降解。停用ATM,CK1或SCFβ-TrCP通过小分子抑制剂或基因敲除/敲除消除了TOP2β的降解。从生物学上讲,TOP2β降解的阻断结合VM-26处理会损害DNA损伤反应和修复,从而导致细胞通过凋亡加速死亡。因此,看来TOP2β降解是促进DSB暴露以触发DNA损伤反应和修复的细胞防御机制。总的来说,我们的发现揭示了一种新的策略,可与小分子抑制剂联合使用以提高TOP2中毒的功效,以对抗TOP2β降解。通过凋亡导致加速的细胞死亡。因此,看来TOP2β降解是促进DSB暴露以触发DNA损伤反应和修复的细胞防御机制。总的来说,我们的发现揭示了一种新的策略,可与小分子抑制剂联合使用以提高TOP2中毒的功效,以对抗TOP2β降解。通过凋亡导致加速的细胞死亡。因此,看来TOP2β降解是促进DSB暴露以触发DNA损伤反应和修复的细胞防御机制。总的来说,我们的发现揭示了一种新的策略,可与小分子抑制剂联合使用以提高TOP2中毒的功效,以对抗TOP2β降解。
更新日期:2020-02-03
中文翻译:

SCFβ-TrCP介导的TOP2β降解可响应靶向拓扑异构酶II的化学治疗药物促进癌细胞存活。
靶向拓扑异构酶II(TOP2)的抗癌化学治疗药物,称为TOP2毒物,通过稳定TOP2-DNA共价复合物以诱导DNA双链断裂(DSB)并最终导致细胞死亡,在临床上得到广泛使用和有效。已知稳定的TOP2-DNA复合物可被蛋白酶体降解,而响应TOP2毒物而导致的即时TOP2β降解的基本机制以及随后的生物学后果仍然难以捉摸。在这里,我们报道了TOP2毒物诱导的TOP2β降解是由SCFβ-TrCP泛素连接酶介导的。具体而言,由替尼泊苷(VM-26)处理触发的DNA损伤信号激活ATM,并与CK1协同作用以规范化的德格伦基序磷酸化Ser1134和Ser1130上的TOP2β,以促进β-TrCP结合和随后的降解。停用ATM,CK1或SCFβ-TrCP通过小分子抑制剂或基因敲除/敲除消除了TOP2β的降解。从生物学上讲,TOP2β降解的阻断结合VM-26处理会损害DNA损伤反应和修复,从而导致细胞通过凋亡加速死亡。因此,看来TOP2β降解是促进DSB暴露以触发DNA损伤反应和修复的细胞防御机制。总的来说,我们的发现揭示了一种新的策略,可与小分子抑制剂联合使用以提高TOP2中毒的功效,以对抗TOP2β降解。通过凋亡导致加速的细胞死亡。因此,看来TOP2β降解是促进DSB暴露以触发DNA损伤反应和修复的细胞防御机制。总的来说,我们的发现揭示了一种新的策略,可与小分子抑制剂联合使用以提高TOP2中毒的功效,以对抗TOP2β降解。通过凋亡导致加速的细胞死亡。因此,看来TOP2β降解是促进DSB暴露以触发DNA损伤反应和修复的细胞防御机制。总的来说,我们的发现揭示了一种新的策略,可与小分子抑制剂联合使用以提高TOP2中毒的功效,以对抗TOP2β降解。































 京公网安备 11010802027423号
京公网安备 11010802027423号