当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Investigation of Isonitrile Formation Catalyzed by the Nonheme Iron/α-KG-Depcs9b05411endent Decarboxylase (ScoE)
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-02-14 , DOI: 10.1021/acscatal.9b05411 Hong Li 1 , Yongjun Liu 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-02-14 , DOI: 10.1021/acscatal.9b05411 Hong Li 1 , Yongjun Liu 1
Affiliation
|
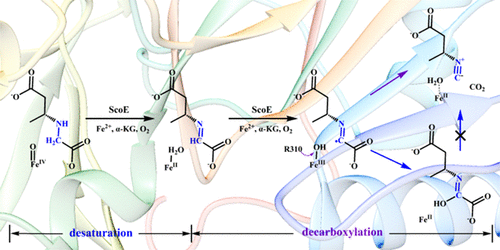
Recent structural and biochemical evidence showed that ScoE from Streptomyces coeruleorubidus is a nonheme iron/α-KG-dependent decarboxylase, which catalyzes the formation of isonitrile group by desaturation and decarboxylation. This discovery offers an alternative mechanism for isonitrile formation. The other isonitrile synthases, such as IsnA, XnPvcA, or AmbI1/AmbI2, convert R–CH(−NH2)–CO2– to R–CH(−N≡C)–CO2– by introducing an additional carbon unit; however, ScoE catalyzes the conversion of R–NH–CH2–CO2– to R–N≡C through oxidative decarboxylation. To explore the catalytic mechanism of ScoE, on the basis of the high-resolution crystal structure, the enzyme–substrate complex models were constructed and a series of combined QM/MM calculations were performed. Our results reveal that the ScoE-catalyzed reaction contains two decoupled parts, desaturation and decarboxylation. The FeIV-oxo-triggered desaturation includes two consecutive H-abstractions, which are similar to the C–C single bond desaturation catalyzed by other nonheme iron/α-KG-dependent desaturases. In the second stage reaction, the decarboxylation of the substrate radical generated by H-abstraction was calculated to be quite easy, whereas the previously proposed decarboxylation that involves the hydroxylated intermediate was calculated to be difficult. Importantly, the electron transfer from the substrate to the iron center is the key factor for lowering the barrier of decarboxylation. Thus, the central iron ion is not only responsible for H-abstraction but also acts as an electron relay station for decarboxylation. In addition, this electron transfer was found to be coupled with a proton transfer, in which R310 and the associated H-bonding network play a critical role. In general, the first C–N desaturation is the rate-limiting step of the whole catalysis with an overall energy barrier of 17.6 or 16.9 kcal/mol in two competitive pathways, qualitatively agreeing with the estimated free energy (17.9–18.1 kcal/mol) from experiments. These results may provide useful information for understanding the biosynthesis of isonitrile and the oxidative decarboxylation catalyzed by nonheme iron/α-KG-dependent enzymes.
中文翻译:

非血红素铁/α-KG-Depcs9b05411末端脱羧酶(ScoE)催化异腈形成的机理研究
最近的结构和生化证据表明,来自蓝藻链霉菌的ScoE是一种非血红素铁/α-KG依赖性脱羧酶,可通过去饱和和脱羧作用催化异腈基团的形成。这一发现为异腈形成提供了另一种机制。其他异腈合成酶(例如IsnA,XnPvcA或AmbI1 / AmbI2)通过引入额外的碳单元,将R–CH(-NH 2)–CO 2 –转化为R–CH(–N≡C)–CO 2 –。然而,ScoE催化R–NH–CH 2 –CO 2 –的转化。通过氧化脱羧反应生成R–N≡C。为了探索ScoE的催化机理,在高分辨率晶体结构的基础上,构建了酶-底物复合物模型,并进行了一系列组合的QM / MM计算。我们的结果表明,ScoE催化的反应包含两个去耦部分:去饱和和去羧化。铁四-氧代触发的去饱和包括两个连续的H-抽象,类似于由其他非血红素铁/α-KG依赖性去饱和酶催化的C–C单键去饱和。在第二阶段反应中,计算出通过氢吸收产生的底物自由基的脱羧相当容易,而先前提出的涉及羟基化中间体的脱羧却很难计算。重要的是,电子从基体转移到铁中心是降低脱羧势垒的关键因素。因此,中心铁离子不仅负责氢的吸收,而且还用作脱羧的电子中继站。此外,发现这种电子转移与质子转移有关,其中R310和相关的H键网络起着至关重要的作用。通常,第一个C–N脱饱和是整个催化的限速步骤,在两个竞争途径中的总能垒为17.6或16.9 kcal / mol,在质量上与估计的自由能(17.9–18.1 kcal / mol)一致)来自实验。这些结果可能为理解异腈的生物合成和非血红素铁/α-KG依赖性酶催化的氧化脱羧提供有用的信息。
更新日期:2020-02-14
中文翻译:

非血红素铁/α-KG-Depcs9b05411末端脱羧酶(ScoE)催化异腈形成的机理研究
最近的结构和生化证据表明,来自蓝藻链霉菌的ScoE是一种非血红素铁/α-KG依赖性脱羧酶,可通过去饱和和脱羧作用催化异腈基团的形成。这一发现为异腈形成提供了另一种机制。其他异腈合成酶(例如IsnA,XnPvcA或AmbI1 / AmbI2)通过引入额外的碳单元,将R–CH(-NH 2)–CO 2 –转化为R–CH(–N≡C)–CO 2 –。然而,ScoE催化R–NH–CH 2 –CO 2 –的转化。通过氧化脱羧反应生成R–N≡C。为了探索ScoE的催化机理,在高分辨率晶体结构的基础上,构建了酶-底物复合物模型,并进行了一系列组合的QM / MM计算。我们的结果表明,ScoE催化的反应包含两个去耦部分:去饱和和去羧化。铁四-氧代触发的去饱和包括两个连续的H-抽象,类似于由其他非血红素铁/α-KG依赖性去饱和酶催化的C–C单键去饱和。在第二阶段反应中,计算出通过氢吸收产生的底物自由基的脱羧相当容易,而先前提出的涉及羟基化中间体的脱羧却很难计算。重要的是,电子从基体转移到铁中心是降低脱羧势垒的关键因素。因此,中心铁离子不仅负责氢的吸收,而且还用作脱羧的电子中继站。此外,发现这种电子转移与质子转移有关,其中R310和相关的H键网络起着至关重要的作用。通常,第一个C–N脱饱和是整个催化的限速步骤,在两个竞争途径中的总能垒为17.6或16.9 kcal / mol,在质量上与估计的自由能(17.9–18.1 kcal / mol)一致)来自实验。这些结果可能为理解异腈的生物合成和非血红素铁/α-KG依赖性酶催化的氧化脱羧提供有用的信息。

































 京公网安备 11010802027423号
京公网安备 11010802027423号