当前位置:
X-MOL 学术
›
JAMA Pediatr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effectiveness of Intrapleural Tissue Plasminogen Activator and Dornase Alfa vs Tissue Plasminogen Activator Alone in Children with Pleural Empyema
JAMA Pediatrics ( IF 24.7 ) Pub Date : 2020-04-01 , DOI: 10.1001/jamapediatrics.2019.5863 Michael H Livingston 1, 2 , Sanjay Mahant 3 , Bairbre Connolly 4 , Ian MacLusky 5 , Sophie Laberge 6 , Lucia Giglia 1 , Connie Yang 7 , Ashley Roberts 7 , Anna Shawyer 8 , Mary Brindle 9 , Simon Parsons 9 , Cristina Stoian 9 , J Mark Walton 1 , Kevin E Thorpe 10, 11 , Yang Chen 11 , Fei Zuo 11 , Muhammad Mamdani 12 , Carol Chan 3 , Desmond Loong 13, 14 , Wanrudee Isaranuwatchai 10, 13, 14 , Felix Ratjen 3 , Eyal Cohen 3
JAMA Pediatrics ( IF 24.7 ) Pub Date : 2020-04-01 , DOI: 10.1001/jamapediatrics.2019.5863 Michael H Livingston 1, 2 , Sanjay Mahant 3 , Bairbre Connolly 4 , Ian MacLusky 5 , Sophie Laberge 6 , Lucia Giglia 1 , Connie Yang 7 , Ashley Roberts 7 , Anna Shawyer 8 , Mary Brindle 9 , Simon Parsons 9 , Cristina Stoian 9 , J Mark Walton 1 , Kevin E Thorpe 10, 11 , Yang Chen 11 , Fei Zuo 11 , Muhammad Mamdani 12 , Carol Chan 3 , Desmond Loong 13, 14 , Wanrudee Isaranuwatchai 10, 13, 14 , Felix Ratjen 3 , Eyal Cohen 3
Affiliation
|
Importance
Clinical guidelines recommend that children with pleural empyema be treated with chest tube insertion and intrapleural fibrinolytics. The addition of dornase alfa (DNase) has been reported to improve outcomes in adults but remains unproven in children. Objective
To determine if intrapleural tissue plasminogen activator (tPA) and DNase is more effective than tPA and placebo at reducing hospital length of stay in children with pleural empyema. Design, Setting, and Participants
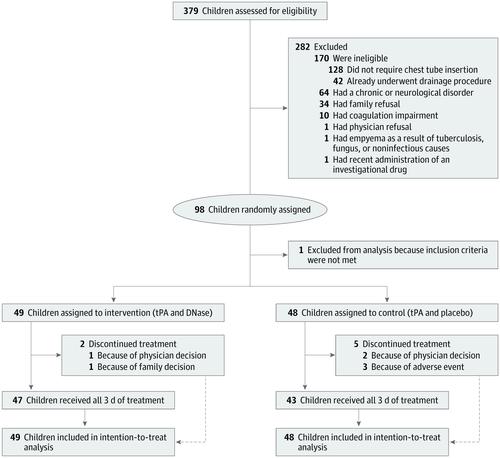
This multicenter, parallel-group, placebo-controlled, superiority randomized clinical trial included children diagnosed as having pleural empyema requiring drainage aged 6 months to 18 years treated at 6 tertiary Canadian children's hospitals. A total of 379 children were assessed for eligibility; 281 were excluded and 98 were randomized. One child was excluded after randomization for not meeting the inclusion criteria. Data were collected from March 4, 2013, to December 13, 2017. Interventions
Participants underwent chest tube insertion and 3 daily administrations of intrapleural tPA, 4 mg, followed by DNase, 5 mg (intervention group), or 5 mL of normal saline (placebo; control group). Participants, families, clinical staff, and members of the study team were blinded to allocation. Main Outcomes and Measures
The primary outcome was hospital length of stay from chest tube insertion to discharge. Secondary outcomes included time to meeting discharge criteria, time to chest tube removal, mean fever duration, additional pleural drainage procedures, hospital readmissions, and total health care cost. Results
Of the 97 analyzed children with pleural empyema, 52 (54%) were male, and the mean (SD) age was 5.1 (3.6) years. A total of 49 children were randomized to tPA and DNase and 48 were randomized to tPA and placebo. Treatment with tPA and DNase was not associated with decreased hospital length of stay compared with tPA and placebo (mean [SD] length of stay, 9.0 [4.9] vs 9.1 [5.3] days; mean difference, -0.1 days; 95% CI, -2.0 to 2.1; P = .96). Similarly, no significant differences were observed for any of the secondary outcomes. Of the 14 adverse events in the tPA and DNase group, 6 (43%) were serious; of the 21 adverse events in the tPA and placebo group, 8 (38%) were serious. There were no deaths. Conclusions and Relevance
The addition of DNase to intrapleural tPA for children with pleural empyema had no effect on hospital length of stay or other outcomes compared with tPA with placebo. Clinical practice guidelines should continue to support the use of chest tube insertion and intrapleural fibrinolytics alone as first-line treatment for pediatric empyema. Trial Registration
ClinicalTrials.gov identifier: NCT01717742.
中文翻译:

胸膜内组织纤溶酶原激活剂和 Dornase Alfa 与单独使用组织纤溶酶原激活剂在儿童胸膜积脓中的有效性
重要性 临床指南建议对患有胸膜积脓的儿童进行胸管插入和胸膜内溶栓治疗。据报道,添加多核糖核酸酶 (DNase) 可以改善成人的结果,但在儿童中仍未得到证实。目的 确定胸膜内组织纤溶酶原激活剂 (tPA) 和 DNase 是否比 tPA 和安慰剂更有效地减少胸膜积脓患儿的住院时间。设计、设置和参与者 这项多中心、平行组、安慰剂对照、优效性随机临床试验包括在加拿大 6 家三级儿童医院接受治疗的 6 个月至 18 岁被诊断患有胸膜积脓需要引流的儿童。共评估了 379 名儿童的资格;281 人被排除在外,98 人被随机分组。随机分组后,一名儿童因不符合纳入标准而被排除在外。数据收集时间为 2013 年 3 月 4 日至 2017 年 12 月 13 日。 干预 参与者接受胸管插入和 3 次每日胸腔内 tPA 4 mg 给药,然后是 DNase 5 mg(干预组)或 5 mL 生理盐水(安慰剂;对照组)。参与者、家属、临床工作人员和研究团队成员对分配不知情。主要结果和测量主要结果是从胸管插入到出院的住院时间。次要结果包括达到出院标准的时间、拔除胸管的时间、平均发热持续时间、额外的胸腔引流程序、再入院和总医疗费用。结果 分析的 97 名胸膜积脓患儿中,52 名 (54%) 为男性,平均 (SD) 年龄为 5.1 (3.6) 岁。共有 49 名儿童随机接受 tPA 和 DNase,48 名儿童随机接受 tPA 和安慰剂。与 tPA 和安慰剂相比,tPA 和 DNase 治疗与住院时间缩短无关(平均 [SD] 住院时间,9.0 [4.9] vs 9.1 [5.3] 天;平均差异,-0.1 天;95% CI, -2.0 到 2.1;P = .96)。同样,任何次要结果均未观察到显着差异。在 tPA 和 DNase 组的 14 起不良事件中,有 6 起(43%)是严重的;在 tPA 和安慰剂组的 21 起不良事件中,有 8 起(38%)是严重的。没有死亡。结论和相关性 与 tPA 和安慰剂相比,在胸膜内 tPA 中添加 DNase 对患有胸膜积脓的儿童对住院时间或其他结果没有影响。临床实践指南应继续支持单独使用胸管插入和胸膜内溶栓作为小儿脓胸的一线治疗。试验注册 ClinicalTrials.gov 标识符:NCT01717742。
更新日期:2020-04-01
中文翻译:

胸膜内组织纤溶酶原激活剂和 Dornase Alfa 与单独使用组织纤溶酶原激活剂在儿童胸膜积脓中的有效性
重要性 临床指南建议对患有胸膜积脓的儿童进行胸管插入和胸膜内溶栓治疗。据报道,添加多核糖核酸酶 (DNase) 可以改善成人的结果,但在儿童中仍未得到证实。目的 确定胸膜内组织纤溶酶原激活剂 (tPA) 和 DNase 是否比 tPA 和安慰剂更有效地减少胸膜积脓患儿的住院时间。设计、设置和参与者 这项多中心、平行组、安慰剂对照、优效性随机临床试验包括在加拿大 6 家三级儿童医院接受治疗的 6 个月至 18 岁被诊断患有胸膜积脓需要引流的儿童。共评估了 379 名儿童的资格;281 人被排除在外,98 人被随机分组。随机分组后,一名儿童因不符合纳入标准而被排除在外。数据收集时间为 2013 年 3 月 4 日至 2017 年 12 月 13 日。 干预 参与者接受胸管插入和 3 次每日胸腔内 tPA 4 mg 给药,然后是 DNase 5 mg(干预组)或 5 mL 生理盐水(安慰剂;对照组)。参与者、家属、临床工作人员和研究团队成员对分配不知情。主要结果和测量主要结果是从胸管插入到出院的住院时间。次要结果包括达到出院标准的时间、拔除胸管的时间、平均发热持续时间、额外的胸腔引流程序、再入院和总医疗费用。结果 分析的 97 名胸膜积脓患儿中,52 名 (54%) 为男性,平均 (SD) 年龄为 5.1 (3.6) 岁。共有 49 名儿童随机接受 tPA 和 DNase,48 名儿童随机接受 tPA 和安慰剂。与 tPA 和安慰剂相比,tPA 和 DNase 治疗与住院时间缩短无关(平均 [SD] 住院时间,9.0 [4.9] vs 9.1 [5.3] 天;平均差异,-0.1 天;95% CI, -2.0 到 2.1;P = .96)。同样,任何次要结果均未观察到显着差异。在 tPA 和 DNase 组的 14 起不良事件中,有 6 起(43%)是严重的;在 tPA 和安慰剂组的 21 起不良事件中,有 8 起(38%)是严重的。没有死亡。结论和相关性 与 tPA 和安慰剂相比,在胸膜内 tPA 中添加 DNase 对患有胸膜积脓的儿童对住院时间或其他结果没有影响。临床实践指南应继续支持单独使用胸管插入和胸膜内溶栓作为小儿脓胸的一线治疗。试验注册 ClinicalTrials.gov 标识符:NCT01717742。

































 京公网安备 11010802027423号
京公网安备 11010802027423号