当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamic Implications of the Ligand Exchange with Alkylamines on the Surface of CdSe Quantum Dots: The Importance of Ligand–Ligand Interactions
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-02-14 , DOI: 10.1021/acs.jpcc.9b11572 Meng Liu 1 , Ying-Ying Wang 1 , Yi Liu 1, 2, 3 , Feng-Lei Jiang 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-02-14 , DOI: 10.1021/acs.jpcc.9b11572 Meng Liu 1 , Ying-Ying Wang 1 , Yi Liu 1, 2, 3 , Feng-Lei Jiang 1
Affiliation
|
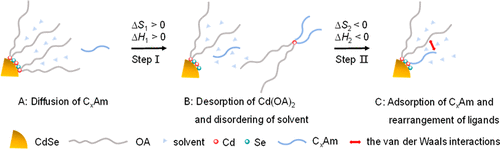
Ligand exchange is a critical step for tuning the properties of quantum dots (QDs), as well as functionalization for wide applications. Previous studies have categorized X-, L-, and Z-types of ligands and classified the ligand exchange process accordingly. Many works have investigated the influences of ligand exchange on the optical properties of QDs. To date, however, the thermodynamic implications of ligand exchange are not well understood, and the mechanisms of distinct effects of the ligands of the same type are unclear. To address these issues, ligand exchange reactions of the oleate-capped CdSe QDs with alkylamines of different carbon chain length, namely, n-butylamine (C4Am), n-octylamine (C8Am), n-dodecylamine (C12Am), were investigated using 1H nuclear magnetic resonance (1H NMR) spectroscopy, fluorescence spectroscopy, X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FTIR), etc. 1H NMR studies showed that the equilibrium constants (Keq) for the desorption of the Z-type ligand (cadmium oleate) from the surface of CdSe QDs, as promoted by the L-type ligands (alkylamines), were in the order of C4Am > C8Am > C12Am, presumably highlighting the small steric barrier of ligands with short carbon chain. In addition, the ligand exchange was studied by fluorescence titration at varied temperatures from a perspective of ligand adsorption following the desorption of cadmium oleate. The binding constants were of magnitude 103 M–1. A “two-step ligand exchange” (TSLE) model was developed to describe the ligand desorption–adsorption process with thermodynamic perspectives. The increased length of the carbon chain brought not only increasing steric barrier but also increasing van der Waals interactions, exhibiting a double-edged sword effect in ligand exchange. The ligand exchange with C8Am contributed the most negative value of ΔH and ΔS since the van der Waals interactions between C8Am and neighboring ligands were stronger than that for C4Am, whereas its steric barrier was weaker than that of C12Am. These demonstrated the importance of ligand–ligand interactions, relatively less documented in the previous studies, besides the predominance of the nanocrystal–ligand interactions. UV–vis absorption spectroscopic and fluorescence lifetime studies further showed that the ligand exchange with all three alkylamines affected optical properties of CdSe QDs to some extent. Powder X-ray diffraction (XRD) and transmission electron microscopy (TEM) results showed that the ligand exchange process basically did not affect the crystal structure and core size of the CdSe QDs. Our work had proposed a conceivable mechanism for ligand exchange, elucidated the thermodynamic implications, and provided a reasonable guidance for selection of suitable ligands.
中文翻译:

CdSe量子点表面上的烷基胺与配体交换的热力学含义:配体-配体相互作用的重要性。
配体交换是调节量子点(QD)特性以及实现广泛应用功能化的关键步骤。先前的研究已对X型,L型和Z型配体进行了分类,并据此对配体交换过程进行了分类。许多工作已经研究了配体交换对量子点光学性质的影响。然而,迄今为止,对配体交换的热力学含义尚不十分了解,并且相同类型配体的不同作用机理尚不清楚。为了解决这些问题,油酸酯封端的CdSe QD与不同碳链长度的烷基胺(即正丁胺(C 4 Am),正辛胺(C 8 Am),n)的配体交换反应-十二烷基胺(C 12 Am),使用1 H核磁共振(1 H NMR)光谱,荧光光谱,X射线光电子能谱(XPS),傅里叶变换红外光谱(FTIR)等进行了研究。1 H NMR研究表明由L型配体(烷基胺)促进的Z型配体(油酸镉)从CdSe量子点表面解吸的平衡常数(K eq)为C 4 Am> C上午8点> C 12Am,大概是突出了具有短碳链的配体的小空间位阻。此外,从油酸镉解吸后的配体吸附的角度出发,通过在不同温度下的荧光滴定研究了配体交换。结合常数为10 3 M –1。建立了“两步配体交换”(TSLE)模型,以热力学观点描述了配体解吸-吸附过程。碳链长度的增加不仅增加了空间位阻,而且增加了范德华相互作用,在配体交换中表现出双刃剑效应。与C 8 Am的配体交换贡献了ΔH和ΔS的最大负值因为C 8 Am与相邻配体之间的范德华相互作用比C 4 Am强,而其空间屏障却比C 12弱上午。这些证明了配体-配体相互作用的重要性,除了纳米晶体-配体相互作用的优势外,在以前的研究中文献记载相对较少。紫外可见吸收光谱和荧光寿命研究进一步表明,与所有三种烷基胺的配体交换在一定程度上影响了CdSe量子点的光学性能。粉末X射线衍射(XRD)和透射电子显微镜(TEM)结果表明,配体交换过程基本上不影响CdSe量子点的晶体结构和核尺寸。我们的工作提出了可能的配体交换机制,阐明了热力学含义,并为选择合适的配体提供了合理的指导。
更新日期:2020-02-14
中文翻译:

CdSe量子点表面上的烷基胺与配体交换的热力学含义:配体-配体相互作用的重要性。
配体交换是调节量子点(QD)特性以及实现广泛应用功能化的关键步骤。先前的研究已对X型,L型和Z型配体进行了分类,并据此对配体交换过程进行了分类。许多工作已经研究了配体交换对量子点光学性质的影响。然而,迄今为止,对配体交换的热力学含义尚不十分了解,并且相同类型配体的不同作用机理尚不清楚。为了解决这些问题,油酸酯封端的CdSe QD与不同碳链长度的烷基胺(即正丁胺(C 4 Am),正辛胺(C 8 Am),n)的配体交换反应-十二烷基胺(C 12 Am),使用1 H核磁共振(1 H NMR)光谱,荧光光谱,X射线光电子能谱(XPS),傅里叶变换红外光谱(FTIR)等进行了研究。1 H NMR研究表明由L型配体(烷基胺)促进的Z型配体(油酸镉)从CdSe量子点表面解吸的平衡常数(K eq)为C 4 Am> C上午8点> C 12Am,大概是突出了具有短碳链的配体的小空间位阻。此外,从油酸镉解吸后的配体吸附的角度出发,通过在不同温度下的荧光滴定研究了配体交换。结合常数为10 3 M –1。建立了“两步配体交换”(TSLE)模型,以热力学观点描述了配体解吸-吸附过程。碳链长度的增加不仅增加了空间位阻,而且增加了范德华相互作用,在配体交换中表现出双刃剑效应。与C 8 Am的配体交换贡献了ΔH和ΔS的最大负值因为C 8 Am与相邻配体之间的范德华相互作用比C 4 Am强,而其空间屏障却比C 12弱上午。这些证明了配体-配体相互作用的重要性,除了纳米晶体-配体相互作用的优势外,在以前的研究中文献记载相对较少。紫外可见吸收光谱和荧光寿命研究进一步表明,与所有三种烷基胺的配体交换在一定程度上影响了CdSe量子点的光学性能。粉末X射线衍射(XRD)和透射电子显微镜(TEM)结果表明,配体交换过程基本上不影响CdSe量子点的晶体结构和核尺寸。我们的工作提出了可能的配体交换机制,阐明了热力学含义,并为选择合适的配体提供了合理的指导。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号