当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Late-Stage 18O Labeling of Primary Sulfonamides via a Degradation-Reconstruction Pathway.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-01-30 , DOI: 10.1002/chem.202000484
Sean W Reilly 1 , Frank Bennett 1 , Patrick S Fier 1 , Sumei Ren 1 , Neil A Strotman 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-01-30 , DOI: 10.1002/chem.202000484
Sean W Reilly 1 , Frank Bennett 1 , Patrick S Fier 1 , Sumei Ren 1 , Neil A Strotman 1
Affiliation
|
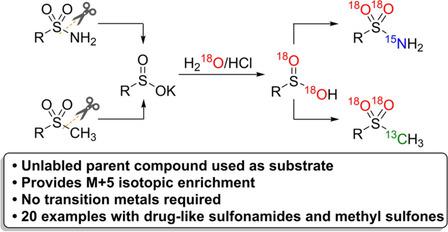
A late-stage 18 O labeling approach of sulfonamides that employs the corresponding unlabeled molecule as the starting material was developed. Upon deamination of the sulfonamide, a sulfinate intermediate was isotopically enriched using eco-friendly reagents H 2 18 O and 15 NH 3 (aq) to afford a M+5 isotopologue of the parent compound. This degradation-reconstruction approach afforded isolated yields of up to 96% for the stable isotope labeled (SIL) sulfonamides, and was compatible with multiple marketed therapeutics, including celecoxib, on a gram scale. The SIL products also exhibited no 18 O/ 16 O back exchange under extreme conditions, further validating the utility of this green strategy for drug labeling for both in vitro and in vivo use. This procedure was also adapted to include pharmaceutically relevant methyl sulfones by using 13 CH 3 , affording M+5 isotopic enrichment, thereby illustrating the broad utility of this methodology.
中文翻译:

通过降解-重建途径对伯磺酰胺进行后期18O标记。
开发了采用相应未标记分子作为起始原料的磺酰胺的后期18 O标记方法。磺酰胺脱氨基后,使用环保试剂H 2 18 O和15 NH 3(aq)同位素富集亚磺酸盐中间体,得到母体化合物的M + 5同位素。这种降解-重建方法可为稳定的同位素标记(SIL)磺酰胺提供高达96%的分离产率,并且与多种市售疗法(包括celecoxib)兼容(以克为单位)。SIL产品在极端条件下也没有18 O / 16 O逆向交换,进一步验证了这种绿色策略在体外和体内药物标记中的实用性。通过使用13 CH 3,该程序也适用于包括药物相关的甲基砜,
更新日期:2020-03-05
中文翻译:

通过降解-重建途径对伯磺酰胺进行后期18O标记。
开发了采用相应未标记分子作为起始原料的磺酰胺的后期18 O标记方法。磺酰胺脱氨基后,使用环保试剂H 2 18 O和15 NH 3(aq)同位素富集亚磺酸盐中间体,得到母体化合物的M + 5同位素。这种降解-重建方法可为稳定的同位素标记(SIL)磺酰胺提供高达96%的分离产率,并且与多种市售疗法(包括celecoxib)兼容(以克为单位)。SIL产品在极端条件下也没有18 O / 16 O逆向交换,进一步验证了这种绿色策略在体外和体内药物标记中的实用性。通过使用13 CH 3,该程序也适用于包括药物相关的甲基砜,































 京公网安备 11010802027423号
京公网安备 11010802027423号