当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reversible interconversion between methanol-diamine and diamide for hydrogen storage based on manganese catalyzed (de)hydrogenation.
Nature Communications ( IF 14.7 ) Pub Date : 2020-01-30 , DOI: 10.1038/s41467-020-14380-3 Zhihui Shao 1 , Yang Li 1, 2 , Chenguang Liu 1 , Wenying Ai 1 , Shu-Ping Luo 2 , Qiang Liu 1, 3
Nature Communications ( IF 14.7 ) Pub Date : 2020-01-30 , DOI: 10.1038/s41467-020-14380-3 Zhihui Shao 1 , Yang Li 1, 2 , Chenguang Liu 1 , Wenying Ai 1 , Shu-Ping Luo 2 , Qiang Liu 1, 3
Affiliation
|
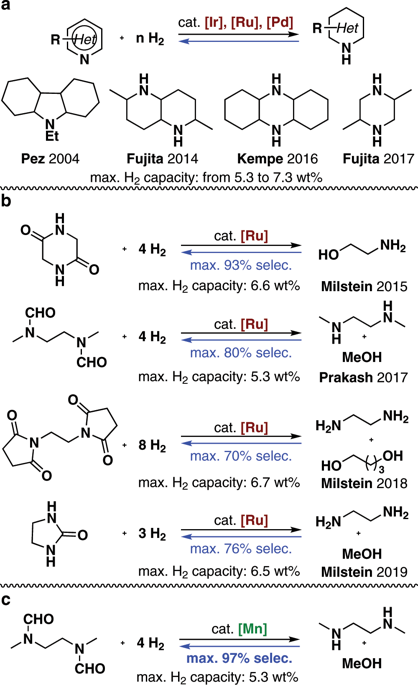
The development of cost-effective, sustainable, and efficient catalysts for liquid organic hydrogen carrier systems is a significant goal. However, all the reported liquid organic hydrogen carrier systems relied on the use of precious metal catalysts. Herein, a liquid organic hydrogen carrier system based on non-noble metal catalysis was established. The Mn-catalyzed dehydrogenative coupling of methanol and N,N'-dimethylethylenediamine to form N,N'-(ethane-1,2-diyl)bis(N-methylformamide), and the reverse hydrogenation reaction constitute a hydrogen storage system with a theoretical hydrogen capacity of 5.3 wt%. A rechargeable hydrogen storage could be achieved by a subsequent hydrogenation of the resulting dehydrogenation mixture to regenerate the H2-rich compound. The maximum selectivity for the dehydrogenative amide formation was 97%.
中文翻译:

基于锰催化的(脱)氢化作用,甲醇-二胺和二酰胺之间可逆的相互转化,用于储氢。
开发用于液态有机氢载体系统的具有成本效益,可持续和高效的催化剂是一个重要目标。然而,所有报道的液态有机氢载体系统都依赖于贵金属催化剂的使用。在此,建立了基于非贵金属催化的液态有机氢载体体系。Mn与N,N'-二甲基乙二胺的Mn催化脱氢反应形成N,N'-(乙烷-1,2-二基)双(N-甲基甲酰胺),逆加氢反应构成了一个氢存储系统理论氢容量为5.3重量%。通过随后对所得脱氢混合物进行氢化以再生富含H 2的化合物,可以实现可再充电的氢存储。脱氢酰胺形成的最大选择性为97%。
更新日期:2020-01-31
中文翻译:

基于锰催化的(脱)氢化作用,甲醇-二胺和二酰胺之间可逆的相互转化,用于储氢。
开发用于液态有机氢载体系统的具有成本效益,可持续和高效的催化剂是一个重要目标。然而,所有报道的液态有机氢载体系统都依赖于贵金属催化剂的使用。在此,建立了基于非贵金属催化的液态有机氢载体体系。Mn与N,N'-二甲基乙二胺的Mn催化脱氢反应形成N,N'-(乙烷-1,2-二基)双(N-甲基甲酰胺),逆加氢反应构成了一个氢存储系统理论氢容量为5.3重量%。通过随后对所得脱氢混合物进行氢化以再生富含H 2的化合物,可以实现可再充电的氢存储。脱氢酰胺形成的最大选择性为97%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号