Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gemcitabine induces Parkin-independent mitophagy through mitochondrial-resident E3 ligase MUL1-mediated stabilization of PINK1.
Scientific Reports ( IF 3.8 ) Pub Date : 2020-01-30 , DOI: 10.1038/s41598-020-58315-w Ryoko Igarashi 1, 2 , Shun-Ichi Yamashita 1 , Tomohiro Yamashita 3 , Keiichi Inoue 1 , Tomoyuki Fukuda 1 , Takeo Fukuchi 2 , Tomotake Kanki 1
Scientific Reports ( IF 3.8 ) Pub Date : 2020-01-30 , DOI: 10.1038/s41598-020-58315-w Ryoko Igarashi 1, 2 , Shun-Ichi Yamashita 1 , Tomohiro Yamashita 3 , Keiichi Inoue 1 , Tomoyuki Fukuda 1 , Takeo Fukuchi 2 , Tomotake Kanki 1
Affiliation
|
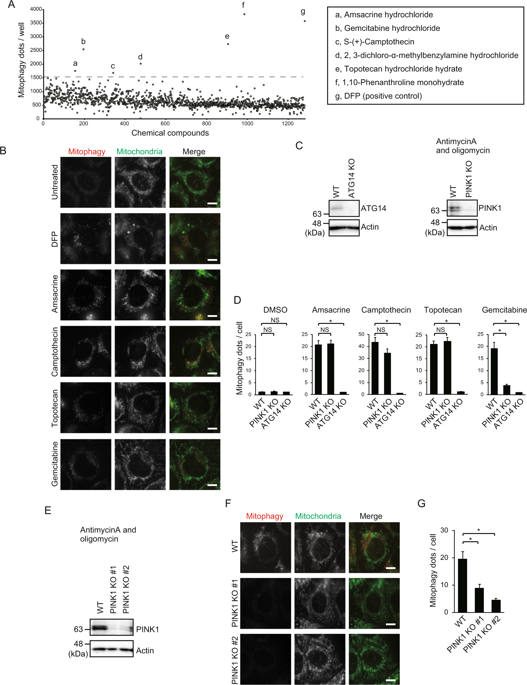
Mitophagy plays an important role in the maintenance of mitochondrial homeostasis. PTEN-induced kinase (PINK1), a key regulator of mitophagy, is degraded constitutively under steady-state conditions. During mitophagy, it becomes stabilized in the outer mitochondrial membrane, particularly under mitochondrial stress conditions, such as in treatment with uncouplers, generation of excessive mitochondrial reactive oxygen species, and formation of protein aggregates in mitochondria. Stabilized PINK1 recruits and activates E3 ligases, such as Parkin and mitochondrial ubiquitin ligase (MUL1), to ubiquitinate mitochondrial proteins and induce ubiquitin-mediated mitophagy. Here, we found that the anticancer drug gemcitabine induces the stabilization of PINK1 and subsequent mitophagy, even in the absence of Parkin. We also found that gemcitabine-induced stabilization of PINK1 was not accompanied by mitochondrial depolarization. Interestingly, the stabilization of PINK1 was mediated by MUL1. These results suggest that gemcitabine induces mitophagy through MUL1-mediated stabilization of PINK1 on the mitochondrial membrane independently of mitochondrial depolarization.
中文翻译:

吉西他滨通过线粒体驻留的E3连接酶MUL1介导的PINK1稳定诱导帕金斯无关的自噬。
线粒体在维持线粒体稳态中起着重要作用。PTEN诱导的激酶(PINK1)是线粒体的关键调节剂,在稳态条件下会组成性降解。在线粒体过程中,它在线粒体外膜中变得稳定,尤其是在线粒体应激条件下,例如在使用解偶联剂处理,产生过多的线粒体活性氧物种以及在线粒体中形成蛋白质聚集体的过程中。稳定的PINK1募集并激活E3连接酶,例如Parkin和线粒体泛素连接酶(MUL1),以泛素化线粒体蛋白并诱导泛素介导的线粒体吞噬。在这里,我们发现抗癌药物吉西他滨即使在没有帕金森氏症的情况下也能诱导PINK1的稳定化和随后的线粒体吞噬。我们还发现吉西他滨诱导的PINK1稳定并不伴随线粒体去极化。有趣的是,PINK1的稳定化是由MUL1介导的。这些结果表明吉西他滨通过线粒体去极化独立于MUL1介导的线粒体膜上PINK1的稳定诱导线粒体。
更新日期:2020-01-31
中文翻译:

吉西他滨通过线粒体驻留的E3连接酶MUL1介导的PINK1稳定诱导帕金斯无关的自噬。
线粒体在维持线粒体稳态中起着重要作用。PTEN诱导的激酶(PINK1)是线粒体的关键调节剂,在稳态条件下会组成性降解。在线粒体过程中,它在线粒体外膜中变得稳定,尤其是在线粒体应激条件下,例如在使用解偶联剂处理,产生过多的线粒体活性氧物种以及在线粒体中形成蛋白质聚集体的过程中。稳定的PINK1募集并激活E3连接酶,例如Parkin和线粒体泛素连接酶(MUL1),以泛素化线粒体蛋白并诱导泛素介导的线粒体吞噬。在这里,我们发现抗癌药物吉西他滨即使在没有帕金森氏症的情况下也能诱导PINK1的稳定化和随后的线粒体吞噬。我们还发现吉西他滨诱导的PINK1稳定并不伴随线粒体去极化。有趣的是,PINK1的稳定化是由MUL1介导的。这些结果表明吉西他滨通过线粒体去极化独立于MUL1介导的线粒体膜上PINK1的稳定诱导线粒体。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号