当前位置:
X-MOL 学术
›
Cell Host Microbe
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Analysis of Rabies Virus Glycoprotein Reveals pH-Dependent Conformational Changes and Interactions with a Neutralizing Antibody.
Cell Host & Microbe ( IF 20.6 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.chom.2019.12.012
Fanli Yang 1 , Sheng Lin 1 , Fei Ye 1 , Jing Yang 1 , Jianxun Qi 2 , Zhujun Chen 1 , Xi Lin 1 , Jichao Wang 1 , Dan Yue 1 , Yanwei Cheng 1 , Zimin Chen 1 , Hua Chen 1 , Yu You 1 , Zhonglin Zhang 1 , Yu Yang 1 , Ming Yang 1 , Honglu Sun 1 , Yuhua Li 3 , Yu Cao 4 , Shengyong Yang 1 , Yuquan Wei 1 , George F Gao 5 , Guangwen Lu 1
Cell Host & Microbe ( IF 20.6 ) Pub Date : 2020-01-23 , DOI: 10.1016/j.chom.2019.12.012
Fanli Yang 1 , Sheng Lin 1 , Fei Ye 1 , Jing Yang 1 , Jianxun Qi 2 , Zhujun Chen 1 , Xi Lin 1 , Jichao Wang 1 , Dan Yue 1 , Yanwei Cheng 1 , Zimin Chen 1 , Hua Chen 1 , Yu You 1 , Zhonglin Zhang 1 , Yu Yang 1 , Ming Yang 1 , Honglu Sun 1 , Yuhua Li 3 , Yu Cao 4 , Shengyong Yang 1 , Yuquan Wei 1 , George F Gao 5 , Guangwen Lu 1
Affiliation
|
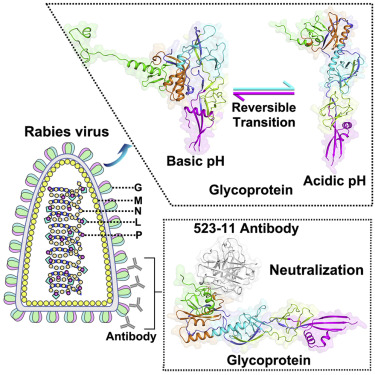
Rabies virus (RABV), the etiological agent for the lethal disease of rabies, is a deadly zoonotic pathogen. The RABV glycoprotein (RABV-G) is a key factor mediating virus entry and the major target of neutralizing antibodies. Here, we report the crystal structures of RABV-G solved in the free form at ∼pH-8.0 and in the complex form with a neutralizing antibody 523-11 at ∼pH-6.5, respectively. RABV-G has three domains, and the basic-to-acidic pH change results in large domain re-orientations and concomitant domain-linker re-constructions, switching it from a bent hairpin conformation into an extended conformation. During such low-pH-induced structural transitions, residues located in the domain-linker are found to play important roles in glycoprotein-mediated membrane fusion. Finally, the antibody interacts with RABV-G mainly through its heavy chain and binds to a bipartite conformational epitope in the viral protein for neutralization. These structures provide valuable information for vaccine and drug design.
中文翻译:

狂犬病病毒糖蛋白的结构分析揭示了pH依赖性构象变化和与中和抗体的相互作用。
狂犬病致死性疾病的病原体狂犬病病毒(RABV)是一种致命的人畜共患病原体。RABV糖蛋白(RABV-G)是介导病毒进入的关键因素,也是中和抗体的主要靶标。在这里,我们报告了RABV-G的晶体结构,分别在〜pH-8.0时以游离形式和在〜pH-6.5时与中和抗体523-11形成复合物。RABV-G具有三个结构域,碱性至酸性pH值变化会导致较大的结构域重新定向和伴随的结构域-接头重建,从而将其从弯曲的发夹构型转变为延伸的构型。在这种低pH诱导的结构转变过程中,发现位于域连接子中的残基在糖蛋白介导的膜融合中起重要作用。最后,该抗体主要通过其重链与RABV-G相互作用,并与病毒蛋白中的两部分构象表位结合以进行中和。这些结构为疫苗和药物设计提供了有价值的信息。
更新日期:2020-01-31
中文翻译:

狂犬病病毒糖蛋白的结构分析揭示了pH依赖性构象变化和与中和抗体的相互作用。
狂犬病致死性疾病的病原体狂犬病病毒(RABV)是一种致命的人畜共患病原体。RABV糖蛋白(RABV-G)是介导病毒进入的关键因素,也是中和抗体的主要靶标。在这里,我们报告了RABV-G的晶体结构,分别在〜pH-8.0时以游离形式和在〜pH-6.5时与中和抗体523-11形成复合物。RABV-G具有三个结构域,碱性至酸性pH值变化会导致较大的结构域重新定向和伴随的结构域-接头重建,从而将其从弯曲的发夹构型转变为延伸的构型。在这种低pH诱导的结构转变过程中,发现位于域连接子中的残基在糖蛋白介导的膜融合中起重要作用。最后,该抗体主要通过其重链与RABV-G相互作用,并与病毒蛋白中的两部分构象表位结合以进行中和。这些结构为疫苗和药物设计提供了有价值的信息。

































 京公网安备 11010802027423号
京公网安备 11010802027423号