当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, biological evaluation and molecular modeling study of 2-amino-3,5-disubstituted-pyrazines as Aurora kinases inhibitors.
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-01-31 , DOI: 10.1016/j.bmc.2020.115351 Yong-Xin Bo 1 , Rong Xiang 2 , Yu Xu 1 , Shu-Yi Hao 1 , Xing-Rong Wang 1 , Shi-Wu Chen 1
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-01-31 , DOI: 10.1016/j.bmc.2020.115351 Yong-Xin Bo 1 , Rong Xiang 2 , Yu Xu 1 , Shu-Yi Hao 1 , Xing-Rong Wang 1 , Shi-Wu Chen 1
Affiliation
|
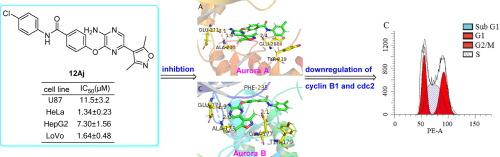
Serine/threonine protein kinases Aurora A, B, and C play essential roles in cell mitosis and cytokinesis, and a number of Aurora kinase inhibitors have been evaluated in the clinic. Herein we report the synthesis and their antiproliferation of 3,5-disubstituted-2-aminopyrazines as kinases inhibitors. Amongst, 4-((3-amino-6- (3,5-dimethylisoxazol-4-yl)pyrazin-2-yl)oxy)-N-(3-chlorophenyl) benzamide (12Aj) exhibited the strongest antiproliferative activities against U38, HeLa, HepG2 and LoVo cells with IC50 values were 11.5 ± 3.2, 1.34 ± 0.23, 7.30 ± 1.56 and 1.64 ± 0.48 μM, as well as inhibited Aurora A and B with the IC50 values were 90 and 152 nM, respectively. Molecular docking studies indicated that 12Aj appeared to form stable hydrogen bonds with either Aurora A or Aurora B. Furthermore, 12Aj arrested HeLa cell cycle in G2/M phase by regulating protein levels of cyclinB1 and cdc2. In addition, the bioinformatics prediction further revealed that 12Aj possessed good drug likeness using SwissADME. These results suggested that 12Aj was worthy of future development of potent anticancer agents as pan-Aurora kinases.
中文翻译:

2-氨基-3,5-二取代吡嗪作为Aurora激酶抑制剂的合成,生物学评估和分子模型研究。
丝氨酸/苏氨酸蛋白激酶Aurora A,B和C在细胞有丝分裂和胞质分裂中起着至关重要的作用,临床上已经评估了许多Aurora激酶抑制剂。在本文中,我们报道了3,5-二取代-2-氨基吡嗪作为激酶抑制剂的合成及其抗增殖作用。其中,4-((3-氨基-6-(3,5-二甲基异恶唑-4-基)吡嗪-2-基)氧基)-N-(3-氯苯基)苯甲酰胺(12Aj)对U38表现出最强的抗增殖活性,具有IC50值的HeLa,HepG2和LoVo细胞分别为11.5±3.2、1.34±0.23、7.30±1.56和1.64±0.48μM,以及抑制的Aurora A和B具有IC50值分别为90和152 nM。分子对接研究表明12Aj似乎与Aurora A或Aurora B形成稳定的氢键。12Aj通过调节cyclinB1和cdc2的蛋白质水平,将HeLa细胞周期阻滞在G2 / M期。此外,生物信息学预测还进一步表明,使用SwissADME,12Aj具有良好的药物相似性。这些结果表明,12Aj值得作为抗泛Aurora激酶的有效抗癌药的未来发展。
更新日期:2020-01-31
中文翻译:

2-氨基-3,5-二取代吡嗪作为Aurora激酶抑制剂的合成,生物学评估和分子模型研究。
丝氨酸/苏氨酸蛋白激酶Aurora A,B和C在细胞有丝分裂和胞质分裂中起着至关重要的作用,临床上已经评估了许多Aurora激酶抑制剂。在本文中,我们报道了3,5-二取代-2-氨基吡嗪作为激酶抑制剂的合成及其抗增殖作用。其中,4-((3-氨基-6-(3,5-二甲基异恶唑-4-基)吡嗪-2-基)氧基)-N-(3-氯苯基)苯甲酰胺(12Aj)对U38表现出最强的抗增殖活性,具有IC50值的HeLa,HepG2和LoVo细胞分别为11.5±3.2、1.34±0.23、7.30±1.56和1.64±0.48μM,以及抑制的Aurora A和B具有IC50值分别为90和152 nM。分子对接研究表明12Aj似乎与Aurora A或Aurora B形成稳定的氢键。12Aj通过调节cyclinB1和cdc2的蛋白质水平,将HeLa细胞周期阻滞在G2 / M期。此外,生物信息学预测还进一步表明,使用SwissADME,12Aj具有良好的药物相似性。这些结果表明,12Aj值得作为抗泛Aurora激酶的有效抗癌药的未来发展。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号