当前位置:
X-MOL 学术
›
J. Pharmaceut. Biomed. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pharmacokinetics, tissue distribution, and safety evaluation of a ligustilide derivative (LIGc).
Journal of Pharmaceutical and Biomedical Analysis ( IF 3.1 ) Pub Date : 2020-01-31 , DOI: 10.1016/j.jpba.2020.113140 Yanxia Zhang 1 , Yaming Zhang 1 , Yanming Han 2 , Ye Tian 2 , Pengcheng Wu 1 , Aiyi Xin 1 , Xiaoning Wei 3 , Yanbin Shi 4 , Zhenchang Zhang 2 , Gang Su 5 , Yanping Shi 3 , Junxi Liu 3
Journal of Pharmaceutical and Biomedical Analysis ( IF 3.1 ) Pub Date : 2020-01-31 , DOI: 10.1016/j.jpba.2020.113140 Yanxia Zhang 1 , Yaming Zhang 1 , Yanming Han 2 , Ye Tian 2 , Pengcheng Wu 1 , Aiyi Xin 1 , Xiaoning Wei 3 , Yanbin Shi 4 , Zhenchang Zhang 2 , Gang Su 5 , Yanping Shi 3 , Junxi Liu 3
Affiliation
|
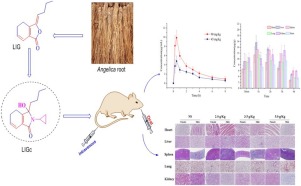
Ligustilide (LIG) is the main active ingredient of Angelica sinensis (Oliv.) Diels and has a neuroprotective effect against ischemic stroke. However, owing to its multi-conjugated unstable structure, the compound has poor drug-forming properties. Therefore, we synthesized highly stable colorless needle crystals (known as ligusticum cycloprolactam, LIGc) through the structural modification of LIG. After a stability experiment was conducted at room temperature for four months, no impurity peaks were found by HPLC-DAD analysis, which indicated that LIGc resolved the stability issues of LIG. LIGc was absorbed and eliminated rapidly after intravenous administration (Cmax = 6.42 ± 1.65 mg/L at a dose of 20 mg/kg) and oral administration (Tmax = 0.5 h, Cmax = 9.89 ± 1.62 mg/L at a dose of 90 mg/kg, t1/2z approximately 2.5 h). The absolute oral bioavailability (F) of LIGc was clearly higher than the F of LIG reported in the literature (F, 83.97 % versus 2.6 %). Linear dose-dependent pharmacokinetics were observed after oral administration, with a higher area under the curve (AUC0-t, 22.31 ± 2.88 mg/L*h) observed at 90 mg/kg than that at 45 mg/kg (AUC0-t, 13.673 ± 0.666 mg/L*h). Tissue distribution results indicated that LIGc easily crossed the blood-brain barrier (BBB) and was distributed widely to the main tissues and organs of rats. We also conducted a preliminary safety assessment of LIGc by means of an acute toxicity test in KM mice. All mice had excellent health status (ig, dosage of 5.0 g/kg), with no histopathological changes observed in the main organs.
中文翻译:

li本内酯衍生物(LIGc)的药代动力学,组织分布和安全性评估。
gust本内酯(LIG)是当归(Oliv。)Diels的主要活性成分,对缺血性中风具有神经保护作用。然而,由于其多缀合的不稳定结构,该化合物具有不良的药物形成特性。因此,我们通过LIG的结构修饰合成了高度稳定的无色针状晶体(称为女贞子环丙内酰胺,LIGc)。在室温下进行四个月的稳定性实验后,通过HPLC-DAD分析未发现任何杂质峰,这表明LIGc解决了LIG的稳定性问题。静脉内给药(Cmax = 6.42±1.65 mg / L,剂量为20 mg / kg)和口服给药(Tmax = 0.5 h,Cmax = 9.89±1.62 mg / L,剂量为90 mg后,LIGc迅速吸收并消除/ kg,t1 / 2z约2.5小时)。LIGc的绝对口服生物利用度(F)明显高于文献中报道的LIG的F(F,83.97%vs. 2.6%)。口服后观察到线性剂量依赖性药代动力学,在90 mg / kg时曲线下面积(AUC0-t,22.31±2.88 mg / L * h)比在45 mg / kg(AUC0-t 13.673±0.666 mg / L * h)。组织分布结果表明,LIGc容易穿过血脑屏障(BBB),并广泛分布于大鼠的主要组织和器官。我们还通过KM小鼠的急性毒性试验对LIGc进行了初步安全性评估。所有小鼠均具有良好的健康状况(ig,剂量为5.0 g / kg),在主要器官中未观察到组织病理学变化。口服后观察到线性剂量依赖性药代动力学,在90 mg / kg时曲线下面积(AUC0-t,22.31±2.88 mg / L * h)比在45 mg / kg(AUC0-t 13.673±0.666 mg / L * h)。组织分布结果表明,LIGc容易穿过血脑屏障(BBB),并广泛分布于大鼠的主要组织和器官。我们还通过KM小鼠的急性毒性试验对LIGc进行了初步安全性评估。所有小鼠均具有良好的健康状况(ig,剂量为5.0 g / kg),在主要器官中未观察到组织病理学变化。口服后观察到线性剂量依赖性药代动力学,在90 mg / kg时曲线下面积(AUC0-t,22.31±2.88 mg / L * h)比在45 mg / kg(AUC0-t 13.673±0.666 mg / L * h)。组织分布结果表明,LIGc容易穿过血脑屏障(BBB),并广泛分布于大鼠的主要组织和器官。我们还通过KM小鼠的急性毒性试验对LIGc进行了初步安全性评估。所有小鼠均具有良好的健康状况(ig,剂量为5.0 g / kg),在主要器官中未观察到组织病理学变化。组织分布结果表明,LIGc容易穿过血脑屏障(BBB),并广泛分布于大鼠的主要组织和器官。我们还通过KM小鼠的急性毒性试验对LIGc进行了初步安全性评估。所有小鼠均具有良好的健康状况(ig,剂量为5.0 g / kg),在主要器官中未观察到组织病理学变化。组织分布结果表明,LIGc容易穿过血脑屏障(BBB),并广泛分布于大鼠的主要组织和器官。我们还通过KM小鼠的急性毒性试验对LIGc进行了初步安全性评估。所有小鼠均具有良好的健康状况(ig,剂量为5.0 g / kg),在主要器官中未观察到组织病理学变化。
更新日期:2020-01-31
中文翻译:

li本内酯衍生物(LIGc)的药代动力学,组织分布和安全性评估。
gust本内酯(LIG)是当归(Oliv。)Diels的主要活性成分,对缺血性中风具有神经保护作用。然而,由于其多缀合的不稳定结构,该化合物具有不良的药物形成特性。因此,我们通过LIG的结构修饰合成了高度稳定的无色针状晶体(称为女贞子环丙内酰胺,LIGc)。在室温下进行四个月的稳定性实验后,通过HPLC-DAD分析未发现任何杂质峰,这表明LIGc解决了LIG的稳定性问题。静脉内给药(Cmax = 6.42±1.65 mg / L,剂量为20 mg / kg)和口服给药(Tmax = 0.5 h,Cmax = 9.89±1.62 mg / L,剂量为90 mg后,LIGc迅速吸收并消除/ kg,t1 / 2z约2.5小时)。LIGc的绝对口服生物利用度(F)明显高于文献中报道的LIG的F(F,83.97%vs. 2.6%)。口服后观察到线性剂量依赖性药代动力学,在90 mg / kg时曲线下面积(AUC0-t,22.31±2.88 mg / L * h)比在45 mg / kg(AUC0-t 13.673±0.666 mg / L * h)。组织分布结果表明,LIGc容易穿过血脑屏障(BBB),并广泛分布于大鼠的主要组织和器官。我们还通过KM小鼠的急性毒性试验对LIGc进行了初步安全性评估。所有小鼠均具有良好的健康状况(ig,剂量为5.0 g / kg),在主要器官中未观察到组织病理学变化。口服后观察到线性剂量依赖性药代动力学,在90 mg / kg时曲线下面积(AUC0-t,22.31±2.88 mg / L * h)比在45 mg / kg(AUC0-t 13.673±0.666 mg / L * h)。组织分布结果表明,LIGc容易穿过血脑屏障(BBB),并广泛分布于大鼠的主要组织和器官。我们还通过KM小鼠的急性毒性试验对LIGc进行了初步安全性评估。所有小鼠均具有良好的健康状况(ig,剂量为5.0 g / kg),在主要器官中未观察到组织病理学变化。口服后观察到线性剂量依赖性药代动力学,在90 mg / kg时曲线下面积(AUC0-t,22.31±2.88 mg / L * h)比在45 mg / kg(AUC0-t 13.673±0.666 mg / L * h)。组织分布结果表明,LIGc容易穿过血脑屏障(BBB),并广泛分布于大鼠的主要组织和器官。我们还通过KM小鼠的急性毒性试验对LIGc进行了初步安全性评估。所有小鼠均具有良好的健康状况(ig,剂量为5.0 g / kg),在主要器官中未观察到组织病理学变化。组织分布结果表明,LIGc容易穿过血脑屏障(BBB),并广泛分布于大鼠的主要组织和器官。我们还通过KM小鼠的急性毒性试验对LIGc进行了初步安全性评估。所有小鼠均具有良好的健康状况(ig,剂量为5.0 g / kg),在主要器官中未观察到组织病理学变化。组织分布结果表明,LIGc容易穿过血脑屏障(BBB),并广泛分布于大鼠的主要组织和器官。我们还通过KM小鼠的急性毒性试验对LIGc进行了初步安全性评估。所有小鼠均具有良好的健康状况(ig,剂量为5.0 g / kg),在主要器官中未观察到组织病理学变化。































 京公网安备 11010802027423号
京公网安备 11010802027423号