当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
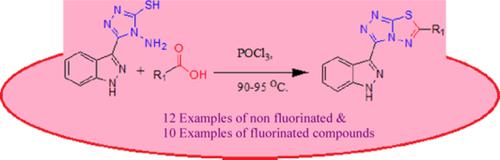
The efficient synthesis of 3‐[6‐(substituted)‐[1,2,4]triazolo[3,4‐b][1,3,4]thiadiazol‐3‐yl]‐1H‐indazole
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-01-28 , DOI: 10.1002/jhet.3866 Santosh Raut 1 , Abdul Hadi 1 , Mohd Arif Pathan 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-01-28 , DOI: 10.1002/jhet.3866 Santosh Raut 1 , Abdul Hadi 1 , Mohd Arif Pathan 1
Affiliation
|
This study presents an efficient synthesis of 3‐[6‐(substituted‐phenyl)‐[1,2,4]triazolo[3,4‐b][1,3,4] thiadiazol‐3‐yl]‐1H‐indazole via dehydrative condensation with cyclization of 4‐amino‐5‐(1H‐indazol‐3‐yl)‐4H‐[1,2,4]triazole‐3‐thiol and fluorinated or nonfluorinated carboxylic acids in presence of phosphorous oxychloride. The multistep reaction pathway proceeds through different compounds. Present synthesis has the advantages of easily accessible starting materials, convenient synthesis, simple reaction condition, wider substrate scope, and higher yield (75% to 90% isolated).
中文翻译:

3- [6-(取代)-[1,2,4]三唑[3,4-b] [1,3,4]噻二唑-3-基] -1H-吲唑的有效合成
这项研究提出3-的有效合成[6-(取代苯基) - [1,2,4]三唑并[3,4- b ] [1,3,4]噻二唑-3-基] -1- ħ -吲哚通过脱水缩合反应与4-氨基-5-(1 H-吲唑-3-基)-4 H- [1,2,4]三唑-3-硫醇和氟化或非氟化羧酸在磷酰氯存在下环化。多步反应途径通过不同的化合物进行。目前的合成方法具有原料易得,合成方便,反应条件简单,底物范围更广,收率更高(分离率为75%至90%)的优点。
更新日期:2020-01-29
中文翻译:

3- [6-(取代)-[1,2,4]三唑[3,4-b] [1,3,4]噻二唑-3-基] -1H-吲唑的有效合成
这项研究提出3-的有效合成[6-(取代苯基) - [1,2,4]三唑并[3,4- b ] [1,3,4]噻二唑-3-基] -1- ħ -吲哚通过脱水缩合反应与4-氨基-5-(1 H-吲唑-3-基)-4 H- [1,2,4]三唑-3-硫醇和氟化或非氟化羧酸在磷酰氯存在下环化。多步反应途径通过不同的化合物进行。目前的合成方法具有原料易得,合成方便,反应条件简单,底物范围更广,收率更高(分离率为75%至90%)的优点。































 京公网安备 11010802027423号
京公网安备 11010802027423号