当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a Scalable Route for a Key Thiadiazole Building Block via Sequential Sandmeyer Bromination and Room-Temperature Suzuki–Miyaura Coupling
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-02-07 , DOI: 10.1021/acs.oprd.9b00495 Gabriel Schäfer 1 , Tony Fleischer 1 , Muhamed Ahmetovic 1 , Stefan Abele 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-02-07 , DOI: 10.1021/acs.oprd.9b00495 Gabriel Schäfer 1 , Tony Fleischer 1 , Muhamed Ahmetovic 1 , Stefan Abele 1
Affiliation
|
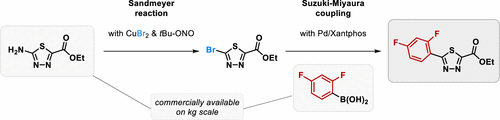
To avoid the use and handling of Lawesson’s reagent or other thiation agents in the in-house kilolab, a new scalable route to ethyl 5-(2,4-difluorophenyl)-1,3,4-thiadiazole-2-carboxylate (1) was developed. The key to success was the use of a commercially available amino-thiadiazole building block, which was converted into the desired product via a sequence of Sandmeyer bromination and Suzuki–Miyaura coupling. The different parameters of the Pd-catalyzed coupling have been studied in detail and allowed the reaction to be performed under mild conditions at room temperature and with low catalyst loading. The inconsistencies of the initial scale-up runs with regard to the sluggish conversion of the Suzuki–Miyaura coupling due to Cu contamination were addressed, and the findings were directly implemented in the subsequent batches, which finally led to an improved overall understanding and robustness of the process.
中文翻译:

通过顺序的沙梅尔溴化和室温的铃木-宫浦联轴器开发关键噻二唑构件的可扩展路线
为避免在内部实验室中使用和处理Lawesson试剂或其他硫代试剂,应采用新的可扩展路线制得5-(2,4-二氟苯基)-1,3,4-噻二唑-2-羧酸乙酯(1) 发展了。成功的关键是使用可商购的氨基噻二唑结构单元,该结构单元通过一系列的Sandmeyer溴化和Suzuki-Miyaura偶联转化为所需的产物。已对Pd催化偶合的不同参数进行了详细研究,并使该反应在温和条件下于室温和低催化剂负载下进行。解决了由于铜污染而导致的铃木-宫浦联轴器转换缓慢的最初扩大操作的不一致之处,并在随后的批次中直接实施了发现,最终导致人们对铜的整体理解和鲁棒性得到了改善过程。
更新日期:2020-02-10
中文翻译:

通过顺序的沙梅尔溴化和室温的铃木-宫浦联轴器开发关键噻二唑构件的可扩展路线
为避免在内部实验室中使用和处理Lawesson试剂或其他硫代试剂,应采用新的可扩展路线制得5-(2,4-二氟苯基)-1,3,4-噻二唑-2-羧酸乙酯(1) 发展了。成功的关键是使用可商购的氨基噻二唑结构单元,该结构单元通过一系列的Sandmeyer溴化和Suzuki-Miyaura偶联转化为所需的产物。已对Pd催化偶合的不同参数进行了详细研究,并使该反应在温和条件下于室温和低催化剂负载下进行。解决了由于铜污染而导致的铃木-宫浦联轴器转换缓慢的最初扩大操作的不一致之处,并在随后的批次中直接实施了发现,最终导致人们对铜的整体理解和鲁棒性得到了改善过程。































 京公网安备 11010802027423号
京公网安备 11010802027423号