Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Silylation of Pyridine, Picolines, and Quinoline with a Zinc Catalyst.
ACS Omega ( IF 3.7 ) Pub Date : 2020-01-10 , DOI: 10.1021/acsomega.9b03317 Joshua W Prybil 1 , Rodney Wallace 1 , Alexandra Warren 1 , Jordan Klingman 1 , Romane Vaillant 2 , Michael B Hall 3 , Xin Yang 3 , William W Brennessel 4 , Robert M Chin 1
ACS Omega ( IF 3.7 ) Pub Date : 2020-01-10 , DOI: 10.1021/acsomega.9b03317 Joshua W Prybil 1 , Rodney Wallace 1 , Alexandra Warren 1 , Jordan Klingman 1 , Romane Vaillant 2 , Michael B Hall 3 , Xin Yang 3 , William W Brennessel 4 , Robert M Chin 1
Affiliation
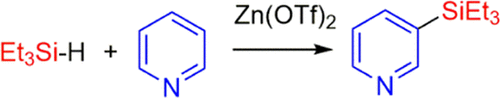
|
Zn(OTf)2 (OTf- = trifluoromethanesulfonate) catalyzes the silylation of pyridine, 3-picoline, and quinoline to afford the silylated products, where the silyl groups are meta to the nitrogen. The isolated yields of the products range from 41 to 26%. The 2- and 4-picolines yielded the silylmethylpyridines, where the CH3 groups were silylated instead of the ring. The pyridine silylation can occur via two separate pathways, involving either a 1,4- or a 1,2-hydrosilylation of pyridine as the first step. A byproduct of the pyridine silylation is a head-to-tail dimerization of N-silyl-1,4-dihydropyridine to form a diazaditwistane molecule.
中文翻译:

用锌催化剂进行吡啶、甲基吡啶和喹啉的硅烷化。
Zn(OTf)2(OTf- = 三氟甲磺酸盐)催化吡啶、3-甲基吡啶和喹啉的甲硅烷基化,得到甲硅烷基化产物,其中甲硅烷基位于氮的间位。产物的分离收率为41%至26%。2-和4-甲基吡啶产生甲硅烷基甲基吡啶,其中CH3基团被甲硅烷基化而不是环。吡啶甲硅烷基化可以通过两个独立的途径发生,第一步涉及吡啶的1,4-或1,2-氢甲硅烷基化。吡啶甲硅烷基化的副产物是 N-甲硅烷基-1,4-二氢吡啶的头尾二聚,形成二氮杂二双烷分子。
更新日期:2020-01-29
中文翻译:

用锌催化剂进行吡啶、甲基吡啶和喹啉的硅烷化。
Zn(OTf)2(OTf- = 三氟甲磺酸盐)催化吡啶、3-甲基吡啶和喹啉的甲硅烷基化,得到甲硅烷基化产物,其中甲硅烷基位于氮的间位。产物的分离收率为41%至26%。2-和4-甲基吡啶产生甲硅烷基甲基吡啶,其中CH3基团被甲硅烷基化而不是环。吡啶甲硅烷基化可以通过两个独立的途径发生,第一步涉及吡啶的1,4-或1,2-氢甲硅烷基化。吡啶甲硅烷基化的副产物是 N-甲硅烷基-1,4-二氢吡啶的头尾二聚,形成二氮杂二双烷分子。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号