Nature ( IF 50.5 ) Pub Date : 2020-01-29 , DOI: 10.1038/s41586-020-1968-7 Sarah van Veen 1 , Shaun Martin 1 , Chris Van den Haute 2, 3 , Veronick Benoy 1 , Joseph Lyons 4 , Roeland Vanhoutte 5 , Jan Pascal Kahler 5 , Jean-Paul Decuypere 1, 6, 7, 8 , Géraldine Gelders 2 , Eric Lambie 9, 10 , Jeffrey Zielich 9 , Johannes V Swinnen 6 , Wim Annaert 7 , Patrizia Agostinis 8, 11 , Bart Ghesquière 12 , Steven Verhelst 5, 13 , Veerle Baekelandt 2 , Jan Eggermont 1 , Peter Vangheluwe 1
|
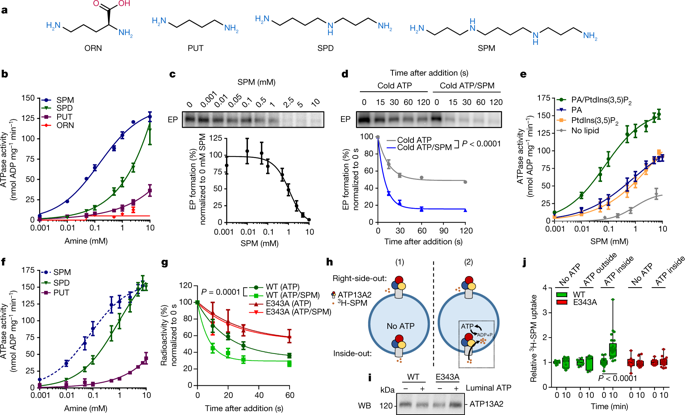
ATP13A2 (PARK9) is a late endolysosomal transporter that is genetically implicated in a spectrum of neurodegenerative disorders, including Kufor-Rakeb syndrome—a parkinsonism with dementia1—and early-onset Parkinson’s disease2. ATP13A2 offers protection against genetic and environmental risk factors of Parkinson’s disease, whereas loss of ATP13A2 compromises lysosomes3. However, the transport function of ATP13A2 in lysosomes remains unclear. Here we establish ATP13A2 as a lysosomal polyamine exporter that shows the highest affinity for spermine among the polyamines examined. Polyamines stimulate the activity of purified ATP13A2, whereas ATP13A2 mutants that are implicated in disease are functionally impaired to a degree that correlates with the disease phenotype. ATP13A2 promotes the cellular uptake of polyamines by endocytosis and transports them into the cytosol, highlighting a role for endolysosomes in the uptake of polyamines into cells. At high concentrations polyamines induce cell toxicity, which is exacerbated by ATP13A2 loss due to lysosomal dysfunction, lysosomal rupture and cathepsin B activation. This phenotype is recapitulated in neurons and nematodes with impaired expression of ATP13A2 or its orthologues. We present defective lysosomal polyamine export as a mechanism for lysosome-dependent cell death that may be implicated in neurodegeneration, and shed light on the molecular identity of the mammalian polyamine transport system.
中文翻译:

ATP13A2 缺乏会破坏溶酶体多胺的输出
ATP13A2 (PARK9) 是一种晚期内溶酶体转运蛋白,在遗传上与一系列神经退行性疾病有关,包括 Kufor-Rakeb 综合征(一种伴有痴呆的帕金森病1)和早发性帕金森病2。ATP13A2 提供对帕金森病遗传和环境风险因素的保护,而 ATP13A2 的缺失会损害溶酶体3. 然而,ATP13A2 在溶酶体中的转运功能仍不清楚。在这里,我们将 ATP13A2 确定为溶酶体多胺出口蛋白,在所检查的多胺中显示出对精胺的最高亲和力。多胺刺激纯化的 ATP13A2 的活性,而与疾病有关的 ATP13A2 突变体的功能受损程度与疾病表型相关。ATP13A2 通过内吞作用促进细胞对多胺的摄取并将它们转运到细胞质中,突出了内溶酶体在将多胺摄取到细胞中的作用。在高浓度下,多胺会诱导细胞毒性,由于溶酶体功能障碍、溶酶体破裂和组织蛋白酶 B 激活导致的 ATP13A2 损失会加剧这种毒性。这种表型在 ATP13A2 或其直系同源物表达受损的神经元和线虫中得到概括。我们提出有缺陷的溶酶体多胺输出作为可能与神经变性有关的溶酶体依赖性细胞死亡的机制,并阐明哺乳动物多胺转运系统的分子特性。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号