当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Hydroxyamidine Based Inhibitors of IDO1 for Cancer Immunotherapy with Reduced Potential for Glucuronidation.
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-01-27 , DOI: 10.1021/acsmedchemlett.9b00572 Christoph Steeneck 1 , Olaf Kinzel 1 , Simon Anderhub 1 , Martin Hornberger 1 , Sheena Pinto 1 , Barbara Morschhaeuser 1 , Floriane Braun 1 , Gerald Kleymann 1 , Thomas Hoffmann 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-01-27 , DOI: 10.1021/acsmedchemlett.9b00572 Christoph Steeneck 1 , Olaf Kinzel 1 , Simon Anderhub 1 , Martin Hornberger 1 , Sheena Pinto 1 , Barbara Morschhaeuser 1 , Floriane Braun 1 , Gerald Kleymann 1 , Thomas Hoffmann 1
Affiliation
|
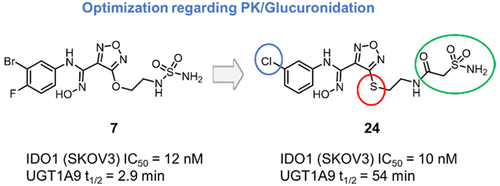
Following the impressive success of checkpoint inhibitors in the treatment of cancer, combinations of IDO1 inhibitors with PD-1/PD-L1 antibodies are in clinical development aiming to increase response rates. Using the hydroxyamidine pharmacophore of the IDO1 inhibitor INCB14943 as a starting point for the design of new inhibitors, the potential shortcomings of extensive hydroxyamidine glucuronidation in humans was addressed. Compounds were optimized using a stability assay with recombinant UGT1A9 enzyme together with the measurement of glucuronide formation in human hepatocytes. Optimized analog 24 showed cellular and biochemical IDO1 IC50 values in the low nanomolar range, a suitable in vitro ADME/PK profile, and efficacy in an animal model of cancer. In a humanized liver mouse model the lead compound exhibited significantly reduced glucuronidation compared to epacadostat (2).
中文翻译:

发现基于羟am啶的IDO1抑制剂可降低癌症糖苷酸化潜力,从而用于癌症免疫治疗。
继检查点抑制剂在治疗癌症方面取得令人瞩目的成功之后,IDO1抑制剂与PD-1 / PD-L1抗体的组合正在临床开发中,旨在提高反应率。以IDO1抑制剂INCB14943的羟am药效团为设计新抑制剂的起点,解决了人体中广泛的羟am葡糖醛酸化的潜在缺点。使用重组UGT1A9酶的稳定性测定法优化化合物,并测量人肝细胞中葡糖醛酸苷的形成。优化的类似物24显示出低纳摩尔范围内的细胞和生化IDO1 IC50值,合适的体外ADME / PK谱以及在癌症动物模型中的功效。
更新日期:2020-01-27
中文翻译:

发现基于羟am啶的IDO1抑制剂可降低癌症糖苷酸化潜力,从而用于癌症免疫治疗。
继检查点抑制剂在治疗癌症方面取得令人瞩目的成功之后,IDO1抑制剂与PD-1 / PD-L1抗体的组合正在临床开发中,旨在提高反应率。以IDO1抑制剂INCB14943的羟am药效团为设计新抑制剂的起点,解决了人体中广泛的羟am葡糖醛酸化的潜在缺点。使用重组UGT1A9酶的稳定性测定法优化化合物,并测量人肝细胞中葡糖醛酸苷的形成。优化的类似物24显示出低纳摩尔范围内的细胞和生化IDO1 IC50值,合适的体外ADME / PK谱以及在癌症动物模型中的功效。






























 京公网安备 11010802027423号
京公网安备 11010802027423号