Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Allosteric Regulation of BH3 Proteins in Bcl-xL Complexes Enables Switch-like Activation of Bax.
Molecular Cell ( IF 14.5 ) Pub Date : 2020-01-27 , DOI: 10.1016/j.molcel.2019.12.025
Christian Bogner 1 , Justin Kale 2 , Justin Pogmore 2 , Xiaoke Chi 2 , Aisha Shamas-Din 3 , Cécile Fradin 4 , Brian Leber 5 , David W Andrews 6
Molecular Cell ( IF 14.5 ) Pub Date : 2020-01-27 , DOI: 10.1016/j.molcel.2019.12.025
Christian Bogner 1 , Justin Kale 2 , Justin Pogmore 2 , Xiaoke Chi 2 , Aisha Shamas-Din 3 , Cécile Fradin 4 , Brian Leber 5 , David W Andrews 6
Affiliation
|
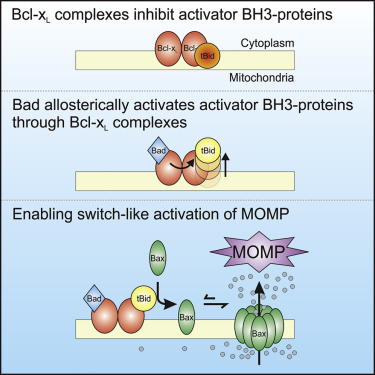
Current models of apoptosis regulation by the Bcl-2 family of proteins postulate that heterodimeric interactions between family members determine whether Bax and Bak are activated to trigger cell death. Thus, the relative abundance and binding affinities between pro- and anti-apoptotic proteins determines the outcome of these interactions. Examination of these interactions using purified mitochondria and liposomes with full-length recombinant proteins revealed that Bcl-xL inhibits apoptosis as a higher-order complex that binds multiple BH3 proteins. Allosteric regulation of this complex by the BH3 sensitizer Bad confers switch-like activity to the indirect activation of Bax. The BH3 activator cBid sequestered by Bcl-xL complexes changes from an inactive to an active form while bound to a Bcl-xL complex only when Bad is also bound. Bcl-xL complexes enable Bad to function as a non-competitive inhibitor of Bcl-xL and allosterically activate cBid, dramatically enhancing the pro-apoptotic potency of Bad.
中文翻译:

Bcl-xL复合物中BH3蛋白的变构调节可启用Bax的开关样激活。
Bcl-2蛋白家族目前的细胞凋亡调控模型假设家族成员之间的异二聚体相互作用决定了Bax和Bak是否被激活以触发细胞死亡。因此,促凋亡蛋白和抗凋亡蛋白之间的相对丰度和结合亲和力决定了这些相互作用的结果。使用纯化的线粒体和脂质体与全长重组蛋白对这些相互作用的检查显示,Bcl-xL作为结合多种BH3蛋白的高级复合物抑制凋亡。BH3敏化剂Bad对该复合物的变构调节将Bads赋予开关样活性。被Bcl-xL复合物螯合的BH3激活剂cBid从非活性形式变为活性形式,而仅当Bad也被结合时才与Bcl-xL复合物结合。
更新日期:2020-01-27
中文翻译:

Bcl-xL复合物中BH3蛋白的变构调节可启用Bax的开关样激活。
Bcl-2蛋白家族目前的细胞凋亡调控模型假设家族成员之间的异二聚体相互作用决定了Bax和Bak是否被激活以触发细胞死亡。因此,促凋亡蛋白和抗凋亡蛋白之间的相对丰度和结合亲和力决定了这些相互作用的结果。使用纯化的线粒体和脂质体与全长重组蛋白对这些相互作用的检查显示,Bcl-xL作为结合多种BH3蛋白的高级复合物抑制凋亡。BH3敏化剂Bad对该复合物的变构调节将Bads赋予开关样活性。被Bcl-xL复合物螯合的BH3激活剂cBid从非活性形式变为活性形式,而仅当Bad也被结合时才与Bcl-xL复合物结合。



































 京公网安备 11010802027423号
京公网安备 11010802027423号