当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ring-Strain Effects in Base-Induced Sommelet-Hauser Rearrangement: Application to Successive Stereocontrolled Transformations
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2016-06-27 , DOI: 10.1002/ejoc.201600611 Eiji Tayama 1 , Kazutoshi Watanabe 1 , Yoshihiro Matano 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2016-06-27 , DOI: 10.1002/ejoc.201600611 Eiji Tayama 1 , Kazutoshi Watanabe 1 , Yoshihiro Matano 1
Affiliation
|
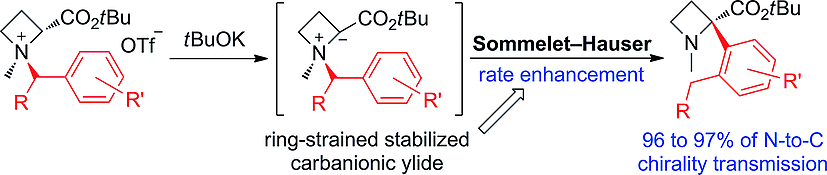
The base-induced Sommelet–Hauser (S–H) rearrangement of azetidine-2-carboxylic acid ester-derived ammonium salts into 2-aryl-substituted derivatives was demonstrated. The ring-strain of four-membered N-heterocycles enables efficient generation of the desired ylide intermediate and enhances the rate of the S–H rearrangement. The asymmetric version of the rearrangement was characterized by excellent levels of successive chirality transmissions. The regio- and stereo-controlled nucleophilic ring opening of the rearrangement products produced quaternary α-aryl amino acid esters with excellent enantiopurities.
中文翻译:

碱基诱导 Sommelet-Hauser 重排中的环应变效应:应用于连续立体控制变换
证明了碱诱导的 Sommelet-Hauser (S-H) 重排氮杂环丁烷-2-羧酸酯衍生的铵盐为 2-芳基取代的衍生物。四元 N-杂环的环应变能够有效生成所需的叶立德中间体并提高 S-H 重排的速率。重排的不对称版本的特点是连续手性传输的水平非常好。重排产物的区域和立体控制的亲核开环产生具有优异对映纯度的季α-芳基氨基酸酯。
更新日期:2016-06-27
中文翻译:

碱基诱导 Sommelet-Hauser 重排中的环应变效应:应用于连续立体控制变换
证明了碱诱导的 Sommelet-Hauser (S-H) 重排氮杂环丁烷-2-羧酸酯衍生的铵盐为 2-芳基取代的衍生物。四元 N-杂环的环应变能够有效生成所需的叶立德中间体并提高 S-H 重排的速率。重排的不对称版本的特点是连续手性传输的水平非常好。重排产物的区域和立体控制的亲核开环产生具有优异对映纯度的季α-芳基氨基酸酯。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号