当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, in vitro, and in vivo (Zebra fish) antitubercular activity of 7,8-dihydroquinolin-5(6H)-ylidenehydrazinecarbothioamides.
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-01-25 , DOI: 10.1016/j.bioorg.2020.103626 Sandeep Kumar Marvadi 1 , Vagolu Siva Krishna 2 , Goverdhan Surineni 1 , Rudraraju Srilakshmi Reshma 2 , Balasubramanian Sridhar 3 , Dharmarajan Sriram 2 , Srinivas Kantevari 4
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-01-25 , DOI: 10.1016/j.bioorg.2020.103626 Sandeep Kumar Marvadi 1 , Vagolu Siva Krishna 2 , Goverdhan Surineni 1 , Rudraraju Srilakshmi Reshma 2 , Balasubramanian Sridhar 3 , Dharmarajan Sriram 2 , Srinivas Kantevari 4
Affiliation
|
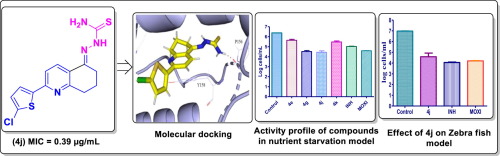
We, herein, describe the synthesis of a series of novel aryl tethered 7,8-dihydroquinolin-5(6H)-ylidenehydrazinecarbothioamides 4a-v, which showed in vitro and in vivo antimycobacterial activity against Mycobacterium tuberculosis (Mtb) H37Rv. The intermediates dihydro-6H-quinolin-5-ones 3a-v were synthesized from β-enaminones, reacting with cyclochexane-1,3-dione/5,5-dimethylcyclohexane-1,3-dione and ammonium acetate using a modified Bohlmann-Rahtz reaction conditions. They were further reacted with thiosemicarbazide to give the respective hydrazine carbothioamides 4a-v. All the new analogues 4a-v, were characterized by their NMR and mass spectral data analysis. Among the twenty-two compounds screened for in vitro antimycobacterial activity against Mycobacterium tuberculosis H37Rv (ATCC27294), two compounds, 4e and 4j, exhibited the highest inhibition with an MIC of 0.39 µg/mL. Compounds 4a, 4g, and 4k were found to inhibit Mtb at an MIC of 0.78 µg/mL. Hydrazinecarbothioamides 4a-k, exhibited enhanced activity than dihydroquinolinones 3a-k. The observed increase in potency provides a clear evidence that hydrazinecarbothioamide is a potential pharmacophore, collectively imparting synergistic effect in enhancing antitubercular activity of the dihydroquinolinone core. The in vivo (Zebra fish) antimycobacterial screening of the in vitro active molecules led to the identification of a hit compound, 4j, with significant activity in the Mtb nutrient starvation model (2.2-fold reduction). Docking studies of 4j showed a hydrogen bond with the P156 residue of the protein.
中文翻译:

7,8-dihydroquinolin-5(6H)-yidnehydrazinecarbothioamides的合成,体外和体内(斑马鱼)抗结核活性。
我们在本文中描述了一系列新型的芳基拴系的7,8-二氢喹啉-5(6H)-亚甲基肼基碳硫代酰胺4a-v的合成,该化合物显示了对结核分枝杆菌(Mtb)H37Rv的体外和体内抗分枝杆菌活性。由β-烯胺酮合成中间体二氢-6H-喹啉-5-酮3a-v,并使用修饰的Bohlmann-酯与环己烷-1,3-二酮/ 5,5-二甲基环己烷-1,3-二酮和乙酸铵反应拉兹反应条件。它们进一步与硫代氨基脲反应,得到各自的肼甲硫代酰胺4a-v。所有新的类似物4a-v均通过NMR和质谱数据分析进行了表征。在筛选出的针对结核分枝杆菌H37Rv(ATCC27294)的体外抗分枝杆菌活性的22种化合物中,两种化合物4e和4j,MIC最高,为0.39 µg / mL。发现化合物4a,4g和4k在MIC为0.78 µg / mL时抑制Mtb。肼基碳硫代酰胺4a-k比二氢喹啉酮3a-k表现出增强的活性。观察到的效力增加提供了明确的证据,表明肼基甲硫基酰胺是潜在的药效团,在增强二氢喹啉酮核心的抗结核活性中共同发挥协同作用。体外活性分子的体内(斑马鱼)抗分枝杆菌筛选导致鉴定出命中化合物4j,该化合物在Mtb营养饥饿模型中具有显着活性(降低了2.2倍)。对4j的对接研究显示该蛋白质的P156残基具有氢键。78 µg / mL。肼基碳硫代酰胺4a-k比二氢喹啉酮3a-k表现出增强的活性。观察到的效力增加提供了明确的证据,表明肼基甲硫基酰胺是潜在的药效团,在增强二氢喹啉酮核心的抗结核活性中共同发挥协同作用。体外活性分子的体内(斑马鱼)抗分枝杆菌筛选导致鉴定出命中化合物4j,该化合物在Mtb营养饥饿模型中具有显着活性(降低了2.2倍)。对4j的对接研究显示该蛋白质的P156残基具有氢键。78 µg / mL。肼基碳硫代酰胺4a-k比二氢喹啉酮3a-k表现出增强的活性。观察到的效力增加提供了明确的证据,表明肼基甲硫基酰胺是潜在的药效团,在增强二氢喹啉酮核心的抗结核活性中共同发挥协同作用。体外活性分子的体内(斑马鱼)抗分枝杆菌筛选导致鉴定出命中化合物4j,该化合物在Mtb营养饥饿模型中具有显着活性(降低了2.2倍)。对4j的对接研究显示该蛋白质的P156残基具有氢键。在增强二氢喹啉酮核心的抗结核活性中共同发挥协同作用。体外活性分子的体内(斑马鱼)抗分枝杆菌筛选导致鉴定出命中化合物4j,该化合物在Mtb营养饥饿模型中具有显着活性(降低了2.2倍)。对4j的对接研究表明该蛋白的P156残基具有氢键。在增强二氢喹啉酮核心的抗结核活性中共同发挥协同作用。体外活性分子的体内(斑马鱼)抗分枝杆菌筛选导致鉴定出命中化合物4j,该化合物在Mtb营养饥饿模型中具有显着活性(降低了2.2倍)。对4j的对接研究显示该蛋白质的P156残基具有氢键。
更新日期:2020-01-26
中文翻译:

7,8-dihydroquinolin-5(6H)-yidnehydrazinecarbothioamides的合成,体外和体内(斑马鱼)抗结核活性。
我们在本文中描述了一系列新型的芳基拴系的7,8-二氢喹啉-5(6H)-亚甲基肼基碳硫代酰胺4a-v的合成,该化合物显示了对结核分枝杆菌(Mtb)H37Rv的体外和体内抗分枝杆菌活性。由β-烯胺酮合成中间体二氢-6H-喹啉-5-酮3a-v,并使用修饰的Bohlmann-酯与环己烷-1,3-二酮/ 5,5-二甲基环己烷-1,3-二酮和乙酸铵反应拉兹反应条件。它们进一步与硫代氨基脲反应,得到各自的肼甲硫代酰胺4a-v。所有新的类似物4a-v均通过NMR和质谱数据分析进行了表征。在筛选出的针对结核分枝杆菌H37Rv(ATCC27294)的体外抗分枝杆菌活性的22种化合物中,两种化合物4e和4j,MIC最高,为0.39 µg / mL。发现化合物4a,4g和4k在MIC为0.78 µg / mL时抑制Mtb。肼基碳硫代酰胺4a-k比二氢喹啉酮3a-k表现出增强的活性。观察到的效力增加提供了明确的证据,表明肼基甲硫基酰胺是潜在的药效团,在增强二氢喹啉酮核心的抗结核活性中共同发挥协同作用。体外活性分子的体内(斑马鱼)抗分枝杆菌筛选导致鉴定出命中化合物4j,该化合物在Mtb营养饥饿模型中具有显着活性(降低了2.2倍)。对4j的对接研究显示该蛋白质的P156残基具有氢键。78 µg / mL。肼基碳硫代酰胺4a-k比二氢喹啉酮3a-k表现出增强的活性。观察到的效力增加提供了明确的证据,表明肼基甲硫基酰胺是潜在的药效团,在增强二氢喹啉酮核心的抗结核活性中共同发挥协同作用。体外活性分子的体内(斑马鱼)抗分枝杆菌筛选导致鉴定出命中化合物4j,该化合物在Mtb营养饥饿模型中具有显着活性(降低了2.2倍)。对4j的对接研究显示该蛋白质的P156残基具有氢键。78 µg / mL。肼基碳硫代酰胺4a-k比二氢喹啉酮3a-k表现出增强的活性。观察到的效力增加提供了明确的证据,表明肼基甲硫基酰胺是潜在的药效团,在增强二氢喹啉酮核心的抗结核活性中共同发挥协同作用。体外活性分子的体内(斑马鱼)抗分枝杆菌筛选导致鉴定出命中化合物4j,该化合物在Mtb营养饥饿模型中具有显着活性(降低了2.2倍)。对4j的对接研究显示该蛋白质的P156残基具有氢键。在增强二氢喹啉酮核心的抗结核活性中共同发挥协同作用。体外活性分子的体内(斑马鱼)抗分枝杆菌筛选导致鉴定出命中化合物4j,该化合物在Mtb营养饥饿模型中具有显着活性(降低了2.2倍)。对4j的对接研究表明该蛋白的P156残基具有氢键。在增强二氢喹啉酮核心的抗结核活性中共同发挥协同作用。体外活性分子的体内(斑马鱼)抗分枝杆菌筛选导致鉴定出命中化合物4j,该化合物在Mtb营养饥饿模型中具有显着活性(降低了2.2倍)。对4j的对接研究显示该蛋白质的P156残基具有氢键。






































 京公网安备 11010802027423号
京公网安备 11010802027423号