当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tuning melatonin receptor subtype selectivity in oxadiazolone-based analogues: Discovery of QR2 ligands and NRF2 activators with neurogenic properties.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-01-25 , DOI: 10.1016/j.ejmech.2020.112090
Clara Herrera-Arozamena 1 , Martín Estrada-Valencia 1 , Concepción Pérez 1 , Laura Lagartera 1 , José A Morales-García 2 , Ana Pérez-Castillo 3 , Juan Felipe Franco-Gonzalez 4 , Patrycja Michalska 4 , Pablo Duarte 4 , Rafael León 5 , Manuela G López 4 , Alberto Mills 6 , Federico Gago 6 , Ángel Juan García-Yagüe 7 , Raquel Fernández-Ginés 7 , Antonio Cuadrado 7 , María Isabel Rodríguez-Franco 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-01-25 , DOI: 10.1016/j.ejmech.2020.112090
Clara Herrera-Arozamena 1 , Martín Estrada-Valencia 1 , Concepción Pérez 1 , Laura Lagartera 1 , José A Morales-García 2 , Ana Pérez-Castillo 3 , Juan Felipe Franco-Gonzalez 4 , Patrycja Michalska 4 , Pablo Duarte 4 , Rafael León 5 , Manuela G López 4 , Alberto Mills 6 , Federico Gago 6 , Ángel Juan García-Yagüe 7 , Raquel Fernández-Ginés 7 , Antonio Cuadrado 7 , María Isabel Rodríguez-Franco 1
Affiliation
|
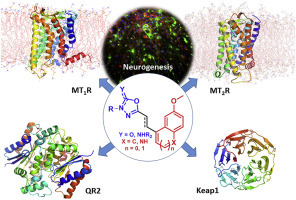
New multi-target indole and naphthalene derivatives containing the oxadiazolone scaffold as a bioisostere of the melatonin acetamido group have been developed. The novel compounds were characterized at melatonin receptors MT1R and MT2R, quinone reductase 2 (QR2), lipoxygenase-5 (LOX-5), and monoamine oxidases (MAO-A and MAO-B), and also as radical scavengers. We found that selectivity within the oxadiazolone series can be modulated by modifying the side chain functionality and co-planarity with the indole or naphthalene ring. In phenotypic assays, several oxadiazolone-based derivatives induced signalling mediated by the transcription factor NRF2 and promoted the maturation of neural stem-cells into a neuronal phenotype. Activation of NRF2 could be due to the binding of indole derivatives to KEAP1, as deduced from surface plasmon resonance (SPR) experiments. Molecular modelling studies using the crystal structures of QR2 and the KEAP1 Kelch-domain, as well as the recently described X-ray free-electron laser (XFEL) structures of chimeric MT1R and MT2R, provided a rationale for the experimental data and afforded valuable insights for future drug design endeavours.
中文翻译:

在基于恶二唑酮的类似物中调节褪黑激素受体亚型的选择性:发现具有神经原性的QR2配体和NRF2激活剂。
已经开发了含有恶二唑酮骨架作为褪黑素乙酰酰胺基团的生物等排体的新的多目标吲哚和萘衍生物。该新型化合物的特征在于褪黑激素受体MT1R和MT2R,醌还原酶2(QR2),脂氧合酶5(LOX-5)和单胺氧化酶(MAO-A和MAO-B),也可作为自由基清除剂。我们发现,可以通过修饰侧链官能度和与吲哚或萘环的共平面度来调节恶二唑酮系列中的选择性。在表型测定中,几种基于恶二唑酮的衍生物诱导由转录因子NRF2介导的信号传导,并促进神经干细胞成熟为神经元表型。NRF2的激活可能是由于吲哚衍生物与KEAP1的结合,由表面等离振子共振(SPR)实验推导得出。使用QR2和KEAP1 Kelch域的晶体结构以及最近描述的嵌合MT1R和MT2R的X射线自由电子激光(XFEL)结构进行分子建模研究,为实验数据提供了理论依据并提供了宝贵的见解用于未来的药物设计。
更新日期:2020-01-26
中文翻译:

在基于恶二唑酮的类似物中调节褪黑激素受体亚型的选择性:发现具有神经原性的QR2配体和NRF2激活剂。
已经开发了含有恶二唑酮骨架作为褪黑素乙酰酰胺基团的生物等排体的新的多目标吲哚和萘衍生物。该新型化合物的特征在于褪黑激素受体MT1R和MT2R,醌还原酶2(QR2),脂氧合酶5(LOX-5)和单胺氧化酶(MAO-A和MAO-B),也可作为自由基清除剂。我们发现,可以通过修饰侧链官能度和与吲哚或萘环的共平面度来调节恶二唑酮系列中的选择性。在表型测定中,几种基于恶二唑酮的衍生物诱导由转录因子NRF2介导的信号传导,并促进神经干细胞成熟为神经元表型。NRF2的激活可能是由于吲哚衍生物与KEAP1的结合,由表面等离振子共振(SPR)实验推导得出。使用QR2和KEAP1 Kelch域的晶体结构以及最近描述的嵌合MT1R和MT2R的X射线自由电子激光(XFEL)结构进行分子建模研究,为实验数据提供了理论依据并提供了宝贵的见解用于未来的药物设计。

































 京公网安备 11010802027423号
京公网安备 11010802027423号