当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper mediated cyclization of 1-substituted enamides, dienamides and trienamides: regiochemistry, indigoid formation and methyl migration-aromatization
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2016-07-01 17:42:38 Joanna V. Geden, Andrew J. Clark, Stuart R. Coles, Collette S. Guy, Franco Ghelfi, Stephen Thom
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2016-07-01 17:42:38 Joanna V. Geden, Andrew J. Clark, Stuart R. Coles, Collette S. Guy, Franco Ghelfi, Stephen Thom
|
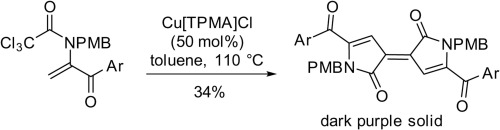
Copper mediated cyclization of activated 1-substituted enamides occurs via a 5-endo radical-polar crossover process. Trichloroacetyl derivatives can undergo further reactions post cyclization (elimination of HCl or dimerization potentially via copper carbenoid intermediates). Reaction of α-halo trienamides derived from β-ionone furnish either β- or γ-lactams via 4-exo or 5-exo cyclizations respectively depending upon the enamide tautomer undergoing reaction. For the less reactive dichloroacetamide derivative a competing regioselective methyl migration-aromatization prior to cyclization is observed.
中文翻译:

铜介导的1-取代的酰胺,二烯酰胺和三烯酰胺的环化:区域化学,靛类形成和甲基迁移-芳香化
铜介导的活化的1-取代的酰胺的环化过程是通过5-内基自由基-极性交叉过程发生的。三氯乙酰基衍生物在环化后可能会进一步反应(消除HCl或可能通过类铜铜中间体二聚化)。衍生自β-紫罗兰酮的α-卤代三烯酰胺的反应分别通过4-exo或5-exo环化提供β-或γ-内酰胺,这取决于所经历的酰胺互变异构体。对于反应性较低的二氯乙酰胺衍生物,观察到在环化之前竞争性的区域选择性甲基迁移-芳香化。
更新日期:2016-07-02
中文翻译:

铜介导的1-取代的酰胺,二烯酰胺和三烯酰胺的环化:区域化学,靛类形成和甲基迁移-芳香化
铜介导的活化的1-取代的酰胺的环化过程是通过5-内基自由基-极性交叉过程发生的。三氯乙酰基衍生物在环化后可能会进一步反应(消除HCl或可能通过类铜铜中间体二聚化)。衍生自β-紫罗兰酮的α-卤代三烯酰胺的反应分别通过4-exo或5-exo环化提供β-或γ-内酰胺,这取决于所经历的酰胺互变异构体。对于反应性较低的二氯乙酰胺衍生物,观察到在环化之前竞争性的区域选择性甲基迁移-芳香化。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号