当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
O-Perfluoropyridin-4-yl Oximes: Iminyl Radical Precursors for Photo- or Thermal-Induced N-O Cleavage in C(sp2)-C(sp3) Bond Formation.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-02-04 , DOI: 10.1021/acs.joc.9b03251 Peng-Ju Xia 1 , Yuan-Zhuo Hu 1 , Zhi-Peng Ye 1 , Xu-Jie Li 1 , Hao-Yue Xiang 1 , Hua Yang 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-02-04 , DOI: 10.1021/acs.joc.9b03251 Peng-Ju Xia 1 , Yuan-Zhuo Hu 1 , Zhi-Peng Ye 1 , Xu-Jie Li 1 , Hao-Yue Xiang 1 , Hua Yang 1, 2
Affiliation
|
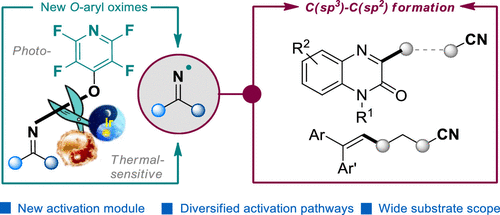
O-Perfluoropyridin-4-yl group was first installed onto cycloketone oximes as a new electrophore, which was proven to be efficient iminyl radical precursors under photocatalytic and thermal conditions. A range of O-perfluoropyridin-4-yl oximes were successfully utilized in C(sp2)-C(sp3) bond formations of quinoxalin-2(1H)-ones and alkenes, providing facile accesses to a range of functionalized alkylnitriles.
中文翻译:

O-Perfluoropyridin-4-yl肟:亚胺基自由基前体的光或热诱导NO裂解C(sp2)-C(sp3)键的形成。
首先,将O-全氟吡啶-4-基作为一种新的电泳试剂安装在环酮肟上,事实证明该试剂是在光催化和热条件下有效的亚氨基自由基前体。一系列O-全氟吡啶-4-基肟已成功用于喹喔啉-2(1H)-一个和烯烃的C(sp2)-C(sp3)键形成中,提供了对一系列功能化烷基腈的便捷连接。
更新日期:2020-02-06
中文翻译:

O-Perfluoropyridin-4-yl肟:亚胺基自由基前体的光或热诱导NO裂解C(sp2)-C(sp3)键的形成。
首先,将O-全氟吡啶-4-基作为一种新的电泳试剂安装在环酮肟上,事实证明该试剂是在光催化和热条件下有效的亚氨基自由基前体。一系列O-全氟吡啶-4-基肟已成功用于喹喔啉-2(1H)-一个和烯烃的C(sp2)-C(sp3)键形成中,提供了对一系列功能化烷基腈的便捷连接。































 京公网安备 11010802027423号
京公网安备 11010802027423号