当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Determination of Hydroxyl Radical Production from Sulfide Oxidation Relevant to Sulfidic Porewaters
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-02-04 , DOI: 10.1021/acsearthspacechem.9b00297 Samuel M. Lombardo 1 , Amanda M. Vindedahl 1 , William A. Arnold 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-02-04 , DOI: 10.1021/acsearthspacechem.9b00297 Samuel M. Lombardo 1 , Amanda M. Vindedahl 1 , William A. Arnold 1
Affiliation
|
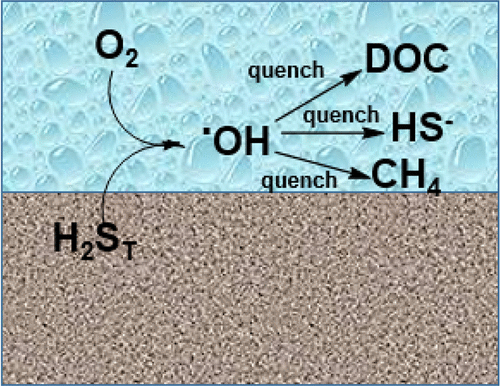
Hydroxyl radical (·OH) production from the reaction between aqueous total sulfide ([H2S]T = [H2S] + [HS–] + [S2–]) and dissolved oxygen is potentially an environmentally important reaction when anoxic, sulfidic water is exposed to oxygen. Using hydroxyterephthalate (hTPA) formation from the reaction of terephthalic acid (TPA) with ·OH as a probe for ·OH production, hydrogen peroxide was verified as an essential intermediate, and the production of free ·OH, versus lower energy hydroxylating agents, was established. The optimal conditions for the quantification of ·OH production kinetics and yield were determined by varying TPA and total sulfide concentrations. An initial total sulfide concentration of 10 μM and TPA concentration of 2 mM was used to find a yield of 15 mmol of ·OH per mole [H2S]T. Additionally, a pseudo-first-order model elucidated a maximum rate of production of 1.04 (±0.05) × 10–4 moles of ·OH per hour per mole of [H2S]T. Experiments with sulfidic wetland porewaters containing up to 294 mg/L of dissolved organic carbon (DOC) revealed that [H2S]T, and not reduced DOC, was the dominant source of ·OH. A simple model considering a water containing [H2S]T, DOC, and methane exposed to a constant concentration of oxygen (∼50 μM) gave steady state values of [·OH] ranging from 5.7 × 10–19 to 3.2 × 10–18 M. The results indicate that [H2S]T should be considered a source of dark formation of ·OH in addition to ferrous iron and reduced DOC.
中文翻译:

测定与硫化物孔隙水有关的硫化物氧化产生的羟基自由基
总硫化物水溶液之间的反应生成羟自由基(·OH)([H 2 S] T = [H 2 S] + [HS – ] + [S 2–])和溶解氧在缺氧,硫化水暴露于氧气时可能对环境具有重要意义。使用由对苯二甲酸(TPA)与·OH反应生成的羟基对苯二甲酸酯(hTPA)作为·OH生成的探针,证实过氧化氢是必不可少的中间体,与低能羟基化剂相比,生成了游离·OH。成立。通过改变TPA和总硫化物的浓度,可以确定定量·OH生产动力学和产率的最佳条件。初始总硫化物浓度为10μM,TPA浓度为2 mM,发现每摩尔[H 2 S] T的产率为15 mmol·OH 。此外,伪一阶模型阐明了最大生产率1.04(±0.05)×10每摩尔[H 2 S] T每小时–4摩尔·OH 。对硫化物湿地孔隙水的实验表明,其溶解有机碳(DOC)高达294 mg / L,表明[H 2 S] T而不是还原的DOC是·OH的主要来源。考虑包含[H 2 S] T,DOC和甲烷的水的简单模型,该水暴露于恒定的氧气浓度(约50μM),其稳态值[·OH]为5.7×10 –19到3.2×10 –18M。结果表明,[H 2 S] T除了亚铁和还原的DOC之外,还应视为·OH深色形成的来源。
更新日期:2020-02-06
中文翻译:

测定与硫化物孔隙水有关的硫化物氧化产生的羟基自由基
总硫化物水溶液之间的反应生成羟自由基(·OH)([H 2 S] T = [H 2 S] + [HS – ] + [S 2–])和溶解氧在缺氧,硫化水暴露于氧气时可能对环境具有重要意义。使用由对苯二甲酸(TPA)与·OH反应生成的羟基对苯二甲酸酯(hTPA)作为·OH生成的探针,证实过氧化氢是必不可少的中间体,与低能羟基化剂相比,生成了游离·OH。成立。通过改变TPA和总硫化物的浓度,可以确定定量·OH生产动力学和产率的最佳条件。初始总硫化物浓度为10μM,TPA浓度为2 mM,发现每摩尔[H 2 S] T的产率为15 mmol·OH 。此外,伪一阶模型阐明了最大生产率1.04(±0.05)×10每摩尔[H 2 S] T每小时–4摩尔·OH 。对硫化物湿地孔隙水的实验表明,其溶解有机碳(DOC)高达294 mg / L,表明[H 2 S] T而不是还原的DOC是·OH的主要来源。考虑包含[H 2 S] T,DOC和甲烷的水的简单模型,该水暴露于恒定的氧气浓度(约50μM),其稳态值[·OH]为5.7×10 –19到3.2×10 –18M。结果表明,[H 2 S] T除了亚铁和还原的DOC之外,还应视为·OH深色形成的来源。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号