当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The proteasome 19S cap and its ubiquitin receptors provide a versatile recognition platform for substrates.
Nature Communications ( IF 14.7 ) Pub Date : 2020-01-24 , DOI: 10.1038/s41467-019-13906-8 Kirby Martinez-Fonts 1 , Caroline Davis 1 , Takuya Tomita 1 , Suzanne Elsasser 2 , Andrew R Nager 3 , Yuan Shi 2 , Daniel Finley 2 , Andreas Matouschek 1
Nature Communications ( IF 14.7 ) Pub Date : 2020-01-24 , DOI: 10.1038/s41467-019-13906-8 Kirby Martinez-Fonts 1 , Caroline Davis 1 , Takuya Tomita 1 , Suzanne Elsasser 2 , Andrew R Nager 3 , Yuan Shi 2 , Daniel Finley 2 , Andreas Matouschek 1
Affiliation
|
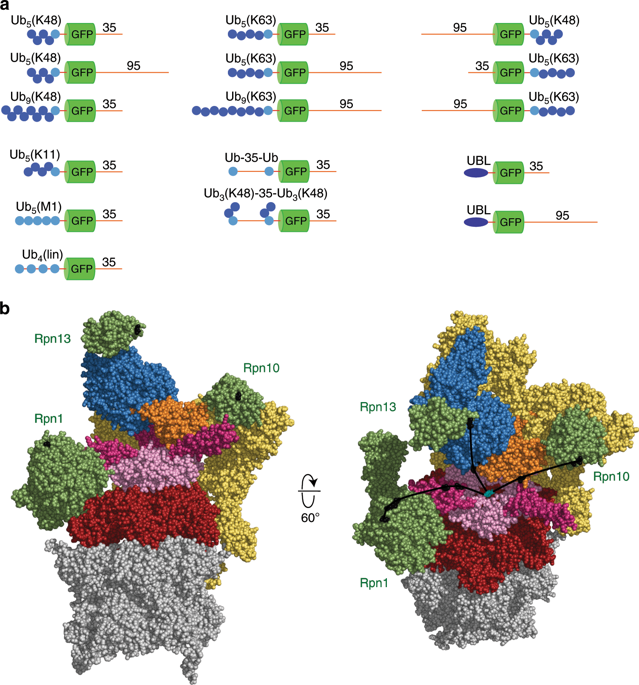
Proteins are targeted to the proteasome by the attachment of ubiquitin chains, which are markedly varied in structure. Three proteasome subunits-Rpn10, Rpn13, and Rpn1-can recognize ubiquitin chains. Here we report that proteins with single chains of K48-linked ubiquitin are targeted for degradation almost exclusively through binding to Rpn10. Rpn1 can act as a co-receptor with Rpn10 for K63 chains and for certain other chain types. Differences in targeting do not correlate with chain affinity to receptors. Surprisingly, in steady-state assays Rpn13 retarded degradation of various single-chain substrates. Substrates with multiple short ubiquitin chains can be presented for degradation by any of the known receptors, whereas those targeted to the proteasome through a ubiquitin-like domain are degraded most efficiently when bound by Rpn13 or Rpn1. Thus, the proteasome provides an unexpectedly versatile binding platform that can recognize substrates targeted for degradation by ubiquitin chains differing greatly in length and topology.
中文翻译:

蛋白酶体19S帽及其泛素受体为底物提供了通用的识别平台。
蛋白质通过泛素链的附着而靶向蛋白酶体,泛素链的结构明显不同。三个蛋白酶体亚基Rpn10,Rpn13和Rpn1-可以识别泛素链。在这里我们报告说,具有单链K48连接的泛素的蛋白质几乎完全通过与Rpn10结合而靶向降解。对于K63链和某些其他链类型,Rpn1可以与Rpn10充当共受体。靶向的差异与链对受体的亲和力无关。出乎意料的是,在稳态测定中,Rpn13抑制了各种单链底物的降解。具有多个短泛素链的底物可被任何已知的受体降解,而通过泛素样结构域靶向蛋白酶体的底物在与Rpn13或Rpn1结合时被最有效地降解。
更新日期:2020-01-24
中文翻译:

蛋白酶体19S帽及其泛素受体为底物提供了通用的识别平台。
蛋白质通过泛素链的附着而靶向蛋白酶体,泛素链的结构明显不同。三个蛋白酶体亚基Rpn10,Rpn13和Rpn1-可以识别泛素链。在这里我们报告说,具有单链K48连接的泛素的蛋白质几乎完全通过与Rpn10结合而靶向降解。对于K63链和某些其他链类型,Rpn1可以与Rpn10充当共受体。靶向的差异与链对受体的亲和力无关。出乎意料的是,在稳态测定中,Rpn13抑制了各种单链底物的降解。具有多个短泛素链的底物可被任何已知的受体降解,而通过泛素样结构域靶向蛋白酶体的底物在与Rpn13或Rpn1结合时被最有效地降解。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号