当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal-Organic Framework in Situ Post-Encapsulating DNA-Enzyme Composites on a Magnetic Carrier with High Stability and Reusability.
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-01-23 , DOI: 10.1021/acsami.9b23526 Zixin Zhou 1 , Zijing Gao 1 , Hao Shen 1 , Mengqi Li 1 , Wenting He 1 , Ping Su 1 , Jiayi Song 1 , Yi Yang 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-01-23 , DOI: 10.1021/acsami.9b23526 Zixin Zhou 1 , Zijing Gao 1 , Hao Shen 1 , Mengqi Li 1 , Wenting He 1 , Ping Su 1 , Jiayi Song 1 , Yi Yang 1
Affiliation
|
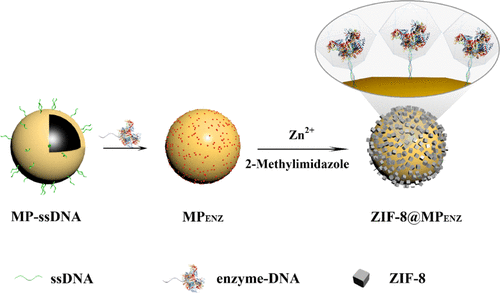
In recent years, metal-organic frameworks (MOFs) have been extensively studied as candidate enzyme immobilization platforms. However, conventional MOF-enzyme composites usually exhibit low controllability and reusability. In this study, a novel and stable strategy for enzyme immobilization was designed by use of ZIF-8 to encapsulate in situ DNA-enzyme composites on the surface of magnetic particles (MPs). The mechanism of in situ encapsulation was discussed in detail. It was found that immobilized enzymes were involved in the growth of ZIF-8, and the DNA cross-linking agents promoted the growth of ZIF-8 on the surface of MP. The thermal, chemical, and physical stabilities of horseradish peroxidase (HRP) were all significantly enhanced after in situ encapsulation. Most importantly, this strategy was proven to be a general platform that can be used to stabilize various proteins. The in situ encapsulation strategy was expanded to immobilize a cascade of enzymes, and ZIF-8@MPGOx-HRP possessed high selectivity and a wide linear range (25-500 μM) for glucose detection.
中文翻译:

具有高稳定性和可重用性的磁性载体上的金属有机骨架原位后包裹DNA酶复合物。
近年来,已广泛研究了金属有机框架(MOF)作为候选酶固定平台。然而,常规的MOF-酶复合物通常表现出低的可控制性和可重复使用性。在这项研究中,通过使用ZIF-8将原位DNA-酶复合物封装在磁性颗粒(MPs)的表面上,设计了一种新颖而稳定的酶固定策略。详细讨论了原位封装的机制。发现固定化酶参与了ZIF-8的生长,并且DNA交联剂促进了ZIF-8在MP表面的生长。原位包封后,辣根过氧化物酶(HRP)的热,化学和物理稳定性均得到显着增强。最重要的是,事实证明,该策略是可用于稳定各种蛋白质的通用平台。扩展了原位包封策略以固定级联的酶,ZIF-8 @ MPGOx-HRP具有高选择性和广泛的线性范围(25-500μM)用于葡萄糖检测。
更新日期:2020-02-04
中文翻译:

具有高稳定性和可重用性的磁性载体上的金属有机骨架原位后包裹DNA酶复合物。
近年来,已广泛研究了金属有机框架(MOF)作为候选酶固定平台。然而,常规的MOF-酶复合物通常表现出低的可控制性和可重复使用性。在这项研究中,通过使用ZIF-8将原位DNA-酶复合物封装在磁性颗粒(MPs)的表面上,设计了一种新颖而稳定的酶固定策略。详细讨论了原位封装的机制。发现固定化酶参与了ZIF-8的生长,并且DNA交联剂促进了ZIF-8在MP表面的生长。原位包封后,辣根过氧化物酶(HRP)的热,化学和物理稳定性均得到显着增强。最重要的是,事实证明,该策略是可用于稳定各种蛋白质的通用平台。扩展了原位包封策略以固定级联的酶,ZIF-8 @ MPGOx-HRP具有高选择性和广泛的线性范围(25-500μM)用于葡萄糖检测。































 京公网安备 11010802027423号
京公网安备 11010802027423号