当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multireference Study of the H2COO (Criegee Intermediate) + O3 Addition: A Reaction of Possible Tropospheric Interest.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-01-22 , DOI: 10.1021/acs.jpca.9b11430 Andrea Maranzana 1 , Glauco Tonachini 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-01-22 , DOI: 10.1021/acs.jpca.9b11430 Andrea Maranzana 1 , Glauco Tonachini 1
Affiliation
|
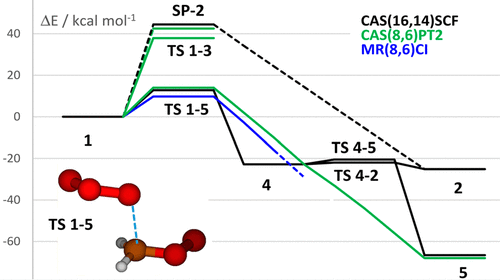
The addition of carbonyl oxides to ozone could have an effect on the tropospheric HO• nocturnal formation. Its mechanistic description has provided so far conflicting results. CASPT2 (and CASSCF) geometry optimizations, focused on the initial addition step, show that the most likely pathway is not a concerted cycloaddition to give directly a c-H2CO5 intermediate, as had been proposed. Concerted cycloaddition is in fact related to a second-order saddle point that puts in turn into relation, obliquely, two distinct diradicaloid transition structures. While O-O addition goes through an energy demanding pathway, C-O addition, potentially producing the open-chain •OOCH2-OOO• diradical intermediate, corresponds to a more viable pathway. CASPT2 and MRCI optimizations, though predicting an addition diradicaloid pathway, describe •OOCH2-OOO• as an unstable structure, because fragmentation to •OOCH2-O• + O2 takes place past the addition TS without any energy barrier: ring closure to c-H2CO5 is thus prevented. The addition E barrier drops as the methods become more demanding, with CASPT3//PT2 at 12.8 kcal mol-1, MRCI at 9.1 kcal mol-1, and MRCC at 4.8 kcal mol-1 (possibly suggesting a significant role of dynamical correlation). Recent experimental data by Chang et al. qualify this addition as a fairly fast reaction.
中文翻译:

H2COO(Criegee中间体)+ O3加成的多参考研究:对流层可能产生的兴趣反应。
向臭氧中添加羰基氧化物可能对对流层HO•夜间形成有影响。迄今为止,其机理描述提供了相互矛盾的结果。侧重于初始添加步骤的CASPT2(和CASSCF)几何优化表明,最可能的途径不是一致的环加成反应,无法直接生成c-H2CO5中间体。协同的环加成实际上与二阶鞍点有关,后者又倾斜地将两个不同的双基过渡结构联系起来。尽管OO加成通过能量需求途径,但CO加成潜在地产生开链•OOCH2-OOO•双自由基中间体,对应于一条更可行的途径。CASPT2和MRCI优化,尽管预测了一条附加的双自由基途径,描述•OOCH2-OOO•为不稳定结构,因为在添加TS之后,•OOCH2-O•+ O2的碎片会发生,而没有任何能垒:因此可以防止c-H2CO5闭环。随着对方法的要求越来越高,附加的E势垒也随之下降,CASPT3 // PT2为12.8 kcal mol-1,MRCI为9.1 kcal mol-1,而MRCC为4.8 kcal mol-1(可能暗示了动力学相关性的重要作用) 。Chang等人的最新实验数据。将此添加视为相当快的反应。8 kcal mol-1(可能暗示了动力学相关性的重要作用)。Chang等人的最新实验数据。将此添加视为相当快的反应。8 kcal mol-1(可能暗示了动力学相关性的重要作用)。Chang等人的最新实验数据。将此添加视为相当快的反应。
更新日期:2020-02-03
中文翻译:

H2COO(Criegee中间体)+ O3加成的多参考研究:对流层可能产生的兴趣反应。
向臭氧中添加羰基氧化物可能对对流层HO•夜间形成有影响。迄今为止,其机理描述提供了相互矛盾的结果。侧重于初始添加步骤的CASPT2(和CASSCF)几何优化表明,最可能的途径不是一致的环加成反应,无法直接生成c-H2CO5中间体。协同的环加成实际上与二阶鞍点有关,后者又倾斜地将两个不同的双基过渡结构联系起来。尽管OO加成通过能量需求途径,但CO加成潜在地产生开链•OOCH2-OOO•双自由基中间体,对应于一条更可行的途径。CASPT2和MRCI优化,尽管预测了一条附加的双自由基途径,描述•OOCH2-OOO•为不稳定结构,因为在添加TS之后,•OOCH2-O•+ O2的碎片会发生,而没有任何能垒:因此可以防止c-H2CO5闭环。随着对方法的要求越来越高,附加的E势垒也随之下降,CASPT3 // PT2为12.8 kcal mol-1,MRCI为9.1 kcal mol-1,而MRCC为4.8 kcal mol-1(可能暗示了动力学相关性的重要作用) 。Chang等人的最新实验数据。将此添加视为相当快的反应。8 kcal mol-1(可能暗示了动力学相关性的重要作用)。Chang等人的最新实验数据。将此添加视为相当快的反应。8 kcal mol-1(可能暗示了动力学相关性的重要作用)。Chang等人的最新实验数据。将此添加视为相当快的反应。






























 京公网安备 11010802027423号
京公网安备 11010802027423号