当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molybdenum-Doped Manganese Oxide as a Highly Efficient and Economical Water Oxidation Catalyst
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-22 , DOI: 10.1021/acscatal.9b02718 S. Esmael Balaghi 1 , C. A. Triana 1 , Greta R. Patzke 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-22 , DOI: 10.1021/acscatal.9b02718 S. Esmael Balaghi 1 , C. A. Triana 1 , Greta R. Patzke 1
Affiliation
|
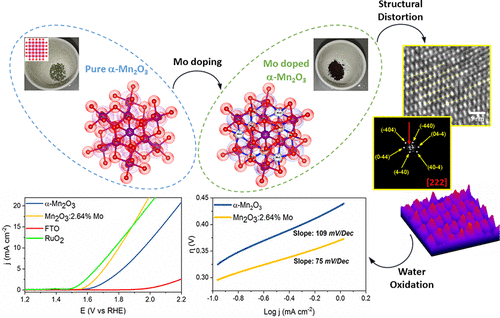
The development of efficient and noble-metal-free electrocatalysts for the challenging oxygen evolution reaction (OER) is crucial for sustainable energy solutions. In this work, a facile co-precipitation method, followed by thermal postsynthetic treatment in N2/air, was developed to synthesize molybdenum-doped α-Mn2O3 materials (Mn2O3:1.72%Mo, Mn2O3:2.64%Mo, Mn2O3:32.23%Mo, and Mn2O3:49.67%Mo) as low-cost water-oxidizing electrocatalysts. Powder X-ray diffraction (PXRD), extended X-ray absorption fine structure (EXAFS), X-ray photoelectron spectroscopy (XPS), and high-resolution transmission electron microscopy (HRTEM) investigations showed the presence of strong distortions in the molybdenum-doped α-Mn2O3 host lattice (Mn2O3:2.64%Mo) and an average oxidation state of Mn2.8+. Several test assays demonstrated that these structural features significantly promote the OER activity. Mn2O3:2.64%Mo was found to exhibit very good activity among the series in cerium ammonium nitrate (CAN)-assisted water oxidation with a maximum turnover frequency (TOF) of 585 μmol O2 m–2 h–1, which is a 15-fold improvement of the pure α-Mn2O3 activity and higher than the value of the previously reported benchmark Mn-based catalyst, birnessite. The optimized catalyst (Mn2O3:2.64%Mo) excelled through a low onset potential (300 mV) and a promising overpotential of 570 mV for OER at a current density of 10 mA cm–2, which is only 20 mV above that of the noble metal benchmark RuO2 electrode and competitive with that of the most active Mn-based OER catalysts reported to date. Electrochemical impedance spectroscopy (EIS) studies demonstrated that the catalytically active surface area of Mn2O3:2.64%Mo is much higher than that of α-Mn2O3 for the OER at the applied potential. In addition, stability during 30 h without degradation was achieved, which exceeds that of a wide range of current noble-metal-free electrocatalysts. Our study provides a facile and effective approach for the preparation of economical and high-performance manganese-based electrocatalysts for water oxidation.
中文翻译:

掺钼氧化锰作为一种高效经济的水氧化催化剂
开发具有挑战性的放氧反应(OER)的高效无贵金属电催化剂对于可持续能源解决方案至关重要。在这项工作中,一个浅显的共沉淀法,随后在N-热合成后处理2 /空气,被开发用于合成钼掺杂α-Mn系2 ö 3种材料(MN 2 ö 3:1.72%的Mo,锰2 ö 3:2.64%Mo,Mn 2 O 3:32.23%Mo和Mn 2 O 3:49.67%Mo)作为低成本的水氧化电催化剂。粉末X射线衍射(PXRD),扩展X射线吸收精细结构(EXAFS),X射线光电子能谱(XPS)和高分辨率透射电子显微镜(HRTEM)研究表明,钼合金中存在强烈的畸变。掺杂α-Mn系2 ö 3主晶格(MN 2 ö 3:2.64%的Mo)和Mn的平均氧化态2.8+。几种测试测定表明,这些结构特征显着促进了OER活性。锰2 O 3:2.64%Mo在硝酸铈铵(CAN)辅助的水氧化中显示出非常好的活性,最大转换频率(TOF)为585μmolO 2 m –2 h –1,即15-折叠纯α-Mn系的改进2 ö 3活性和高于以前报道的基准Mn系催化剂,水钠锰矿的值。经过优化的催化剂(Mn 2 O 3:2.64%Mo)在电流密度为10 mA cm –2的情况下,对于OER具有较低的起始电势(300 mV)和有希望的570 mV过电势,这仅比该值高20 mV,因此表现出色贵金属基准RuO 2电极,并且与迄今为止报道的最具活性的锰基OER催化剂相比具有竞争力。电化学阻抗谱(EIS)的研究表明,Mn的催化活性表面积2 ö 3:2.64%Mo是比α-Mn系的高得多2 ö 3在所施加的电势为OER。另外,获得了在30h内不会降解的稳定性,这超出了目前许多无贵金属的电催化剂的稳定性。我们的研究为制备经济高效的水氧化锰基电催化剂提供了一种简便有效的方法。
更新日期:2020-01-23
中文翻译:

掺钼氧化锰作为一种高效经济的水氧化催化剂
开发具有挑战性的放氧反应(OER)的高效无贵金属电催化剂对于可持续能源解决方案至关重要。在这项工作中,一个浅显的共沉淀法,随后在N-热合成后处理2 /空气,被开发用于合成钼掺杂α-Mn系2 ö 3种材料(MN 2 ö 3:1.72%的Mo,锰2 ö 3:2.64%Mo,Mn 2 O 3:32.23%Mo和Mn 2 O 3:49.67%Mo)作为低成本的水氧化电催化剂。粉末X射线衍射(PXRD),扩展X射线吸收精细结构(EXAFS),X射线光电子能谱(XPS)和高分辨率透射电子显微镜(HRTEM)研究表明,钼合金中存在强烈的畸变。掺杂α-Mn系2 ö 3主晶格(MN 2 ö 3:2.64%的Mo)和Mn的平均氧化态2.8+。几种测试测定表明,这些结构特征显着促进了OER活性。锰2 O 3:2.64%Mo在硝酸铈铵(CAN)辅助的水氧化中显示出非常好的活性,最大转换频率(TOF)为585μmolO 2 m –2 h –1,即15-折叠纯α-Mn系的改进2 ö 3活性和高于以前报道的基准Mn系催化剂,水钠锰矿的值。经过优化的催化剂(Mn 2 O 3:2.64%Mo)在电流密度为10 mA cm –2的情况下,对于OER具有较低的起始电势(300 mV)和有希望的570 mV过电势,这仅比该值高20 mV,因此表现出色贵金属基准RuO 2电极,并且与迄今为止报道的最具活性的锰基OER催化剂相比具有竞争力。电化学阻抗谱(EIS)的研究表明,Mn的催化活性表面积2 ö 3:2.64%Mo是比α-Mn系的高得多2 ö 3在所施加的电势为OER。另外,获得了在30h内不会降解的稳定性,这超出了目前许多无贵金属的电催化剂的稳定性。我们的研究为制备经济高效的水氧化锰基电催化剂提供了一种简便有效的方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号