当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Conversion of Syngas into Tetramethylbenzene via an Aldol-Aromatic Mechanism
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-02-03 , DOI: 10.1021/acscatal.9b03417 Muhammad Tahir Arslan 1 , Babar Ali 1 , Syed Zulfiqar Ali Gilani 1 , Yilin Hou 1 , Qi Wang 2 , Dali Cai 1 , Yao Wang 1 , Fei Wei 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-02-03 , DOI: 10.1021/acscatal.9b03417 Muhammad Tahir Arslan 1 , Babar Ali 1 , Syed Zulfiqar Ali Gilani 1 , Yilin Hou 1 , Qi Wang 2 , Dali Cai 1 , Yao Wang 1 , Fei Wei 1
Affiliation
|
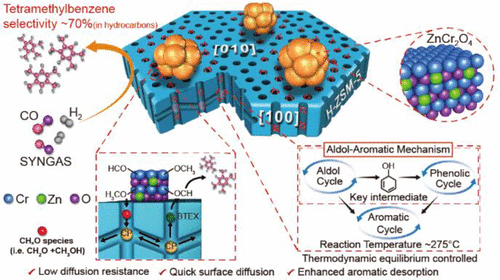
Selectivity control in the single-step conversion of syngas to a single aromatic product is a big challenge. Here, we report an aldol-aromatic mechanism composed of aldol, phenolic, and aromatic cycles, that gave high selectivity >70% of a single product, tetramethylbenzene (TeMB) in hydrocarbons, at a reaction temperature as low as 275 °C. We evidently found the existence of oxygenated-aromatic compounds in the carbon pool, which remained active throughout the reaction and acted as key intermediates for the formation of the aromatics. The physical contact of ZnCr2O4 with H-ZSM-5 exhibited a strong coupling effect that promoted surface diffusion of C1 oxygenates (i.e., formaldehyde and methanol) from ZnCr2O4 into H-ZSM-5 and transformed into aromatics via an aldol-aromatic reaction pathway, thus overcoming the most difficult step for first carbon–carbon bond formation. In addition, ZnCr2O4 promoted the aromatics desorption by lowering the desorption activation energy and prevented the oversaturation of carbon pool species. Furthermore, it was found that a combination of thermodynamic equilibrium, surface methylation, and static repulsion are the key factors for giving high selectivity of TeMB in both carbon pool and final aromatics. This aldol-aromatic mechanism will open an efficient reaction pathway to upscale the process for selective aromatic synthesis in high yield from syngas.
中文翻译:

通过醛醇-芳香族机理选择性地将合成气转化为四甲基苯
合成气一步转化为单一芳族产物的选择性控制是一个巨大的挑战。在这里,我们报道了由羟醛,酚醛和芳香族环组成的羟醛-芳香族机理,该化合物在低至275°C的反应温度下,对烃类中的单产物四甲基苯(TeMB)的选择性高> 70%。我们显然发现碳池中存在氧化芳族化合物,这些化合物在整个反应过程中保持活性,并充当形成芳族化合物的关键中间体。ZnCr 2 O 4与H-ZSM-5的物理接触表现出较强的偶联作用,从而促进了C 1氧化物(即甲醛和甲醇)从ZnCr 2 O 4的表面扩散。进入H-ZSM-5并通过羟醛-芳烃反应途径转化为芳烃,从而克服了第一个碳-碳键形成最困难的步骤。另外,ZnCr 2 O 4通过降低解吸活化能来促进芳族化合物的解吸并防止碳库物质的过饱和。此外,发现热力学平衡,表面甲基化和静态排斥的结合是在碳库和最终芳烃中提供高选择性的TeMB的关键因素。该醛醇-芳族机理将打开有效的反应途径,以规模化从合成气中以高收率选择性合成芳族化合物的方法。
更新日期:2020-02-04
中文翻译:

通过醛醇-芳香族机理选择性地将合成气转化为四甲基苯
合成气一步转化为单一芳族产物的选择性控制是一个巨大的挑战。在这里,我们报道了由羟醛,酚醛和芳香族环组成的羟醛-芳香族机理,该化合物在低至275°C的反应温度下,对烃类中的单产物四甲基苯(TeMB)的选择性高> 70%。我们显然发现碳池中存在氧化芳族化合物,这些化合物在整个反应过程中保持活性,并充当形成芳族化合物的关键中间体。ZnCr 2 O 4与H-ZSM-5的物理接触表现出较强的偶联作用,从而促进了C 1氧化物(即甲醛和甲醇)从ZnCr 2 O 4的表面扩散。进入H-ZSM-5并通过羟醛-芳烃反应途径转化为芳烃,从而克服了第一个碳-碳键形成最困难的步骤。另外,ZnCr 2 O 4通过降低解吸活化能来促进芳族化合物的解吸并防止碳库物质的过饱和。此外,发现热力学平衡,表面甲基化和静态排斥的结合是在碳库和最终芳烃中提供高选择性的TeMB的关键因素。该醛醇-芳族机理将打开有效的反应途径,以规模化从合成气中以高收率选择性合成芳族化合物的方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号