当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic and Crystallographic Studies of Azoreductase AzoA from Bacillus wakoensis A01.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-01-29 , DOI: 10.1021/acschembio.9b00970 Elvira Romero 1 , Simone Savino 1 , Marco W Fraaije 1 , Nikola Lončar 1, 2
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-01-29 , DOI: 10.1021/acschembio.9b00970 Elvira Romero 1 , Simone Savino 1 , Marco W Fraaije 1 , Nikola Lončar 1, 2
Affiliation
|
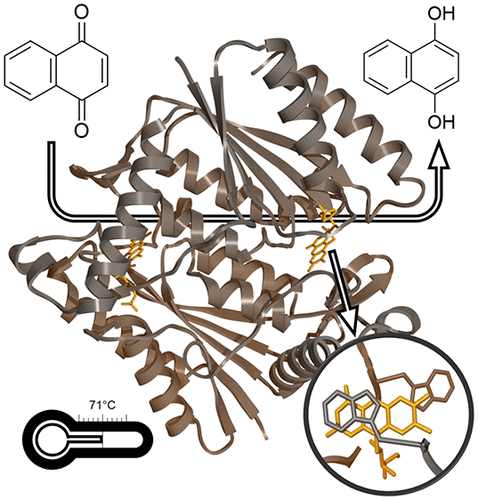
The azoreductase AzoA from the alkali-tolerant Bacillus wakoensis A01 has been studied to reveal its structural and mechanistic details. For this, a recombinant expression system was developed which yields impressive amounts of fully active enzyme. The purified holo enzyme is remarkably solvent-tolerant and thermostable with an apparent melting temperature of 71 °C. The dimeric enzyme contains FMN as a prosthetic group and is strictly NADH dependent. While AzoA shows a negligible ability to use molecular oxygen as an electron acceptor, it is efficient in reducing various azo dyes and quinones. The kinetic and catalytic mechanism has been studied in detail using steady state kinetic analyses and stopped-flow studies. The data show that AzoA performs quinone and azo dye reductions via a two-electron transfer. Moreover, quinones were shown to be much better substrates (kcat values of 100-400 s-1 for several naphtoquinones) when compared with azo dyes. This suggests that the physiological role of AzoA and sequence-related microbial reductases is linked to quinone reductions and that they can better be annotated as quinone reductases. The structure of AzoA has been determined in complex with FMN at 1.8 Å resolution. AzoA displays unique features in the active site providing clues for explaining its catalytic and thermostability features. An uncommon loop, when compared with sequence-related reductases, forms an active site lid with Trp60 acting as an anchor. Several Trp60 mutants have been analyzed disclosing an important role of this residue in the stability of AzoA, while they retained activity. Structural details are discussed in relation to other azo and quinone reductases. This study provides new insights into the molecular functioning of AzoA and sequence-related reductases.
中文翻译:

瓦科芽孢杆菌 A01 偶氮还原酶 AzoA 的机理和晶体学研究。
对来自耐碱芽孢杆菌 A01 的偶氮还原酶 AzoA 进行了研究,以揭示其结构和机制细节。为此,开发了一种重组表达系统,该系统可产生大量完全活性的酶。纯化的 Holo 酶具有出色的溶剂耐受性和热稳定性,表观熔解温度为 71 °C。二聚酶含有 FMN 作为辅基,并且严格依赖 NADH。虽然 AzoA 使用分子氧作为电子受体的能力可以忽略不计,但它可以有效还原各种偶氮染料和醌。使用稳态动力学分析和停流研究详细研究了动力学和催化机制。数据表明,AzoA 通过双电子转移进行醌和偶氮染料还原。此外,与偶氮染料相比,醌被证明是更好的底物(几种萘醌的 kcat 值为 100-400 s-1)。这表明 AzoA 和序列相关微生物还原酶的生理作用与醌还原有关,并且它们可以更好地注释为醌还原酶。已以 1.8 Å 分辨率测定了 AzoA 与 FMN 复合物的结构。 AzoA 在活性位点表现出独特的特征,为解释其催化和热稳定性特征提供了线索。与序列相关还原酶相比,一个不常见的环形成了一个活性位点盖,Trp60 作为锚点。对几种 Trp60 突变体进行了分析,揭示了该残基在 AzoA 稳定性中的重要作用,同时它们保留了活性。讨论了与其他偶氮和醌还原酶相关的结构细节。 这项研究为 AzoA 和序列相关还原酶的分子功能提供了新的见解。
更新日期:2020-01-29
中文翻译:

瓦科芽孢杆菌 A01 偶氮还原酶 AzoA 的机理和晶体学研究。
对来自耐碱芽孢杆菌 A01 的偶氮还原酶 AzoA 进行了研究,以揭示其结构和机制细节。为此,开发了一种重组表达系统,该系统可产生大量完全活性的酶。纯化的 Holo 酶具有出色的溶剂耐受性和热稳定性,表观熔解温度为 71 °C。二聚酶含有 FMN 作为辅基,并且严格依赖 NADH。虽然 AzoA 使用分子氧作为电子受体的能力可以忽略不计,但它可以有效还原各种偶氮染料和醌。使用稳态动力学分析和停流研究详细研究了动力学和催化机制。数据表明,AzoA 通过双电子转移进行醌和偶氮染料还原。此外,与偶氮染料相比,醌被证明是更好的底物(几种萘醌的 kcat 值为 100-400 s-1)。这表明 AzoA 和序列相关微生物还原酶的生理作用与醌还原有关,并且它们可以更好地注释为醌还原酶。已以 1.8 Å 分辨率测定了 AzoA 与 FMN 复合物的结构。 AzoA 在活性位点表现出独特的特征,为解释其催化和热稳定性特征提供了线索。与序列相关还原酶相比,一个不常见的环形成了一个活性位点盖,Trp60 作为锚点。对几种 Trp60 突变体进行了分析,揭示了该残基在 AzoA 稳定性中的重要作用,同时它们保留了活性。讨论了与其他偶氮和醌还原酶相关的结构细节。 这项研究为 AzoA 和序列相关还原酶的分子功能提供了新的见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号