Nature ( IF 50.5 ) Pub Date : 2020-01-22 , DOI: 10.1038/s41586-019-1928-2 Eyal Golub 1 , Rohit H Subramanian 1 , Julian Esselborn 1 , Robert G Alberstein 1 , Jake B Bailey 1 , Jerika A Chiong 1 , Xiaodong Yan 2 , Timothy Booth 2 , Timothy S Baker 2 , F Akif Tezcan 1, 3
|
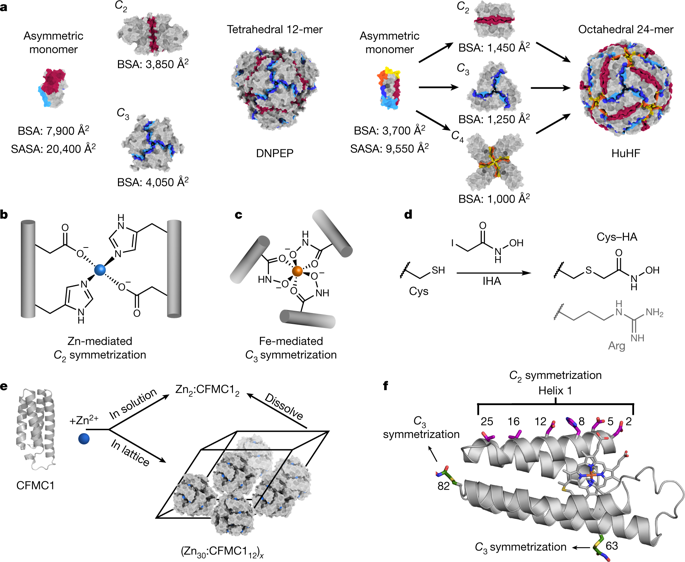
Many proteins exist naturally as symmetrical homooligomers or homopolymers1. The emergent structural and functional properties of such protein assemblies have inspired extensive efforts in biomolecular design2,3,4,5. As synthesized by ribosomes, proteins are inherently asymmetric. Thus, they must acquire multiple surface patches that selectively associate to generate the different symmetry elements needed to form higher-order architectures1,6—a daunting task for protein design. Here we address this problem using an inorganic chemical approach, whereby multiple modes of protein–protein interactions and symmetry are simultaneously achieved by selective, ‘one-pot’ coordination of soft and hard metal ions. We show that a monomeric protein (protomer) appropriately modified with biologically inspired hydroxamate groups and zinc-binding motifs assembles through concurrent Fe3+ and Zn2+ coordination into discrete dodecameric and hexameric cages. Our cages closely resemble natural polyhedral protein architectures7,8 and are, to our knowledge, unique among designed systems9,10,11,12,13 in that they possess tightly packed shells devoid of large apertures. At the same time, they can assemble and disassemble in response to diverse stimuli, owing to their heterobimetallic construction on minimal interprotein-bonding footprints. With stoichiometries ranging from [2 Fe:9 Zn:6 protomers] to [8 Fe:21 Zn:12 protomers], these protein cages represent some of the compositionally most complex protein assemblies—or inorganic coordination complexes—obtained by design.
中文翻译:

通过正交化学相互作用构建蛋白质多面体
许多蛋白质以对称的同源寡聚体或均聚物形式天然存在1。这种蛋白质组装体的新兴结构和功能特性激发了生物分子设计的广泛努力2,3,4,5。由核糖体合成的蛋白质本质上是不对称的。因此,他们必须获得多个表面斑块,这些斑块选择性地结合以产生形成高阶结构所需的不同对称元件1,6——这是蛋白质设计的一项艰巨任务。在这里,我们使用无机化学方法解决了这个问题,其中通过软金属离子和硬金属离子的选择性“一锅法”配位同时实现了蛋白质-蛋白质相互作用和对称性的多种模式。我们表明,用生物启发的异羟肟酸盐基团和锌结合基序适当修饰的单体蛋白(原体)通过并发的 Fe3+ 和 Zn2+ 配位组装成离散的十二聚体和六聚体笼。我们的笼子与天然多面体蛋白结构7,8 非常相似,据我们所知,在设计系统9,10,11,12,13 中是独一无二的,因为它们具有紧密排列的外壳,没有大孔。同时,它们可以响应不同的刺激进行组装和拆卸,因为它们的异双金属结构在最小的蛋白质间键合足迹上。这些蛋白质笼的化学计量范围从 [2 Fe:9 Zn:6 质聚体] 到 [8 Fe:21 Zn:12 质聚体],代表了通过设计获得的一些组成最复杂的蛋白质组装体或无机配位配合物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号