当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A dual-ligand fusion peptide improves the brain-neuron targeting of nanocarriers in Alzheimer's disease mice.
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-01-21 , DOI: 10.1016/j.jconrel.2020.01.039
Qian Guo 1 , Shuting Xu 1 , Peng Yang 1 , Pengzhen Wang 1 , Shuai Lu 2 , Dongyu Sheng 1 , Kang Qian 1 , Jinxu Cao 1 , Wei Lu 1 , Qizhi Zhang 1
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-01-21 , DOI: 10.1016/j.jconrel.2020.01.039
Qian Guo 1 , Shuting Xu 1 , Peng Yang 1 , Pengzhen Wang 1 , Shuai Lu 2 , Dongyu Sheng 1 , Kang Qian 1 , Jinxu Cao 1 , Wei Lu 1 , Qizhi Zhang 1
Affiliation
|
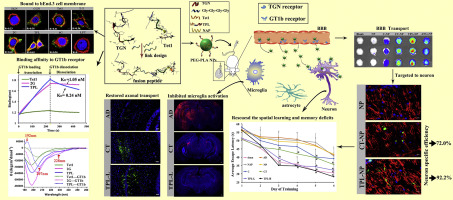
The presence of blood-brain barrier (BBB) and specificity of neuron targeting remain two challenges in the effective delivery of nanotherapeutics for the treatment of Alzheimers disease (AD). Traditional strategy of nanocarriers for AD treatment involves co-decoration of both BBB-penetrating ligand and neuron-targeting ligand on the surface of the nanoparticles for "dual-stage" targeted delivery. Instead, we design and optimize a fusion peptide TPL comprising a BBB-penetrating peptide TGN and a neuron binding peptide Tet1 through a four-glycine linker. Compared to the mono-ligand Tet1 or CGN which is the retro-inverso isomer of TGN with higher brain targeting than TGN, the dual-ligand fusion peptide TPL has preferable blood stability and enhanced structural flexibility, resulting in higher binding affinity to either GT1b ganglioside receptor or brain capillary endothelial bEnd.3 cells. The TPL-modified nanoparticles (TPL-NP) increased the BBB-penetration and neuron-targeting efficacy than the nanoparticles co-decorated with the two mono-ligands. Encapsulation of a neuroprotective peptide NAP, TPL-NP significantly enhance reactive oxygen species scavenging ability and effectively protect microtubule from Aβ25-35-induced neurotoxicity. Meanwhile, TPL-NP inhibit okadaic acid-induced tau aggregation and neuronal apoptosis. Administration of TPL-NP in AD mice also significantly improves the cognitive performance, down-regulates the tau phosphorylation level, promotes axonal transport and attenuates microgliosis. Taken together, this work demonstrates that the rationally designed dual-ligand fusion peptides can greatly improve the delivery of drugs to the AD lesions, thereby markedly enhancing the efficacy of AD treatment.
中文翻译:

双配体融合肽改善了阿尔茨海默氏病小鼠中脑神经元对纳米载体的靶向性。
血脑屏障(BBB)的存在和神经元靶向的特异性仍然是纳米药物有效递送治疗阿尔茨海默氏病(AD)的两个挑战。用于AD治疗的纳米载体的传统策略涉及在纳米颗粒表面上的BBB穿透性配体和神经元靶向性配体的共修饰,以进行“双阶段”靶向递送。相反,我们设计并优化了融合肽TPL,该肽包含BBB穿透肽TGN和神经元结合肽Tet1通过四甘氨酸接头。与单配体Tet1或CGN(TGN的逆向异构体)相比,TGN具有更高的脑靶向性,双配体融合肽TPL具有更好的血液稳定性和增强的结构灵活性,导致与GT1b神经节苷脂受体或脑毛细血管内皮bEnd.3细胞的结合亲和力更高。与用两个单配体共同修饰的纳米粒子相比,TPL修饰的纳米粒子(TPL-NP)增加了BBB渗透和神经元靶向功效。神经保护肽NAP,TPL-NP的封装显着增强了清除活性氧的能力,并有效地保护了微管免受Aβ25-35诱导的神经毒性。同时,TPL-NP抑制冈田酸诱导的tau聚集和神经元凋亡。在AD小鼠中施用TPL-NP还可以显着改善认知能力,下调tau磷酸化水平,促进轴突运输并减轻小胶质细胞增生。在一起
更新日期:2020-01-22
中文翻译:

双配体融合肽改善了阿尔茨海默氏病小鼠中脑神经元对纳米载体的靶向性。
血脑屏障(BBB)的存在和神经元靶向的特异性仍然是纳米药物有效递送治疗阿尔茨海默氏病(AD)的两个挑战。用于AD治疗的纳米载体的传统策略涉及在纳米颗粒表面上的BBB穿透性配体和神经元靶向性配体的共修饰,以进行“双阶段”靶向递送。相反,我们设计并优化了融合肽TPL,该肽包含BBB穿透肽TGN和神经元结合肽Tet1通过四甘氨酸接头。与单配体Tet1或CGN(TGN的逆向异构体)相比,TGN具有更高的脑靶向性,双配体融合肽TPL具有更好的血液稳定性和增强的结构灵活性,导致与GT1b神经节苷脂受体或脑毛细血管内皮bEnd.3细胞的结合亲和力更高。与用两个单配体共同修饰的纳米粒子相比,TPL修饰的纳米粒子(TPL-NP)增加了BBB渗透和神经元靶向功效。神经保护肽NAP,TPL-NP的封装显着增强了清除活性氧的能力,并有效地保护了微管免受Aβ25-35诱导的神经毒性。同时,TPL-NP抑制冈田酸诱导的tau聚集和神经元凋亡。在AD小鼠中施用TPL-NP还可以显着改善认知能力,下调tau磷酸化水平,促进轴突运输并减轻小胶质细胞增生。在一起































 京公网安备 11010802027423号
京公网安备 11010802027423号