Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Constitutive Interferon Attenuates RIPK1/3-Mediated Cytokine Translation.
Cell Reports ( IF 7.5 ) Pub Date : 2020-01-21 , DOI: 10.1016/j.celrep.2019.12.073 Hayley I Muendlein 1 , Joseph Sarhan 2 , Beiyun C Liu 3 , Wilson M Connolly 4 , Stephen A Schworer 5 , Irina Smirnova 4 , Amy Y Tang 4 , Vladimir Ilyukha 6 , Jodie Pietruska 7 , Soroush Tahmasebi 8 , Nahum Sonenberg 8 , Alexei Degterev 7 , Alexander Poltorak 9
Cell Reports ( IF 7.5 ) Pub Date : 2020-01-21 , DOI: 10.1016/j.celrep.2019.12.073 Hayley I Muendlein 1 , Joseph Sarhan 2 , Beiyun C Liu 3 , Wilson M Connolly 4 , Stephen A Schworer 5 , Irina Smirnova 4 , Amy Y Tang 4 , Vladimir Ilyukha 6 , Jodie Pietruska 7 , Soroush Tahmasebi 8 , Nahum Sonenberg 8 , Alexei Degterev 7 , Alexander Poltorak 9
Affiliation
|
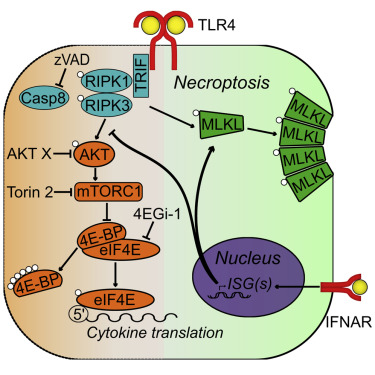
Receptor-interacting protein kinase 1 (RIPK1) and 3 (RIPK3) are well known for their capacity to drive necroptosis via mixed-lineage kinase-like domain (MLKL). Recently, RIPK1/3 kinase activity has been shown to drive inflammation via activation of MAPK signaling. However, the regulatory mechanisms underlying this kinase-dependent cytokine production remain poorly understood. In the present study, we establish that the kinase activity of RIPK1/3 regulates cytokine translation in mouse and human macrophages. Furthermore, we show that this inflammatory response is downregulated by type I interferon (IFN) signaling, independent of type I IFN-promoted cell death. Specifically, low-level constitutive IFN signaling attenuates RIPK-driven activation of cap-dependent translation initiation pathway components AKT, mTORC1, 4E-BP and eIF4E, while promoting RIPK-dependent cell death. Altogether, these data characterize constitutive IFN signaling as a regulator of RIPK-dependent inflammation and establish cap-dependent translation as a crucial checkpoint in the regulation of cytokine production.
中文翻译:

组成型干扰素减弱 RIPK1/3 介导的细胞因子翻译。
受体相互作用蛋白激酶 1 (RIPK1) 和 3 (RIPK3) 因其通过混合谱系激酶样结构域 (MLKL) 驱动坏死性凋亡的能力而闻名。最近,RIPK1/3 激酶活性已被证明可通过激活 MAPK 信号传导来驱动炎症。然而,人们对这种激酶依赖性细胞因子产生的调节机制仍知之甚少。在本研究中,我们确定 RIPK1/3 的激酶活性调节小鼠和人类巨噬细胞中的细胞因子翻译。此外,我们发现这种炎症反应被 I 型干扰素 (IFN) 信号传导下调,与 I 型 IFN 促进的细胞死亡无关。具体来说,低水平的组成型 IFN 信号传导会减弱 RIPK 驱动的帽依赖性翻译起始通路组件 AKT、mTORC1、4E-BP 和 eIF4E 的激活,同时促进 RIPK 依赖性细胞死亡。总而言之,这些数据将组成型 IFN 信号传导描述为 RIPK 依赖性炎症的调节剂,并将帽依赖性翻译确立为细胞因子产生调节中的关键检查点。
更新日期:2020-01-22
中文翻译:

组成型干扰素减弱 RIPK1/3 介导的细胞因子翻译。
受体相互作用蛋白激酶 1 (RIPK1) 和 3 (RIPK3) 因其通过混合谱系激酶样结构域 (MLKL) 驱动坏死性凋亡的能力而闻名。最近,RIPK1/3 激酶活性已被证明可通过激活 MAPK 信号传导来驱动炎症。然而,人们对这种激酶依赖性细胞因子产生的调节机制仍知之甚少。在本研究中,我们确定 RIPK1/3 的激酶活性调节小鼠和人类巨噬细胞中的细胞因子翻译。此外,我们发现这种炎症反应被 I 型干扰素 (IFN) 信号传导下调,与 I 型 IFN 促进的细胞死亡无关。具体来说,低水平的组成型 IFN 信号传导会减弱 RIPK 驱动的帽依赖性翻译起始通路组件 AKT、mTORC1、4E-BP 和 eIF4E 的激活,同时促进 RIPK 依赖性细胞死亡。总而言之,这些数据将组成型 IFN 信号传导描述为 RIPK 依赖性炎症的调节剂,并将帽依赖性翻译确立为细胞因子产生调节中的关键检查点。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号