当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CO2 Solubility in 1,1,2,2-Tetrafluoroethanesulfonate Anion-Based Ionic Liquids: [EMIM][TFES], [BMIM][TFES], and [BNMIM][TFES]
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-01-21 , DOI: 10.1021/acs.jced.9b00833 Joon-Hyuk Yim 1 , Kwang Woo Park 1 , Byung-Keun Oh 1 , Jong Sung Lim 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-01-21 , DOI: 10.1021/acs.jced.9b00833 Joon-Hyuk Yim 1 , Kwang Woo Park 1 , Byung-Keun Oh 1 , Jong Sung Lim 1
Affiliation
|
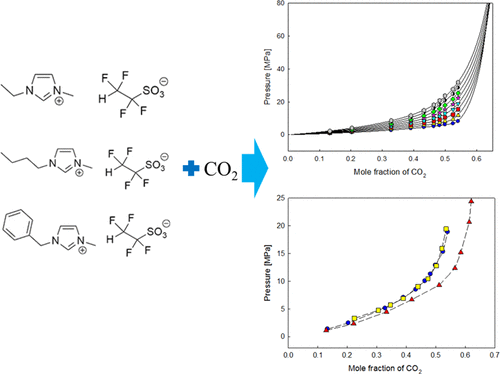
The solubility of carbon dioxide (CO2) in three different ionic liquids (ILs): 1-ethyl-3-methylimidazolium 1,1,2,2-tetrafluoroethanesulfonate ([EMIM][TFES]), 1-butyl-3-methylimidazolium 1,1,2,2-tetrafluoroethanesulfonate ([BMIM][TFES]), and 1-benzyl-3-methylimidazolium 1,1,2,2-tetrafluoroethanesulfonate ([BNMIM][TFES]) were measured. Measurements were done at temperatures ranging from 303.2 to 373.2 K (323.2 to 373.2 K for [BNMIM][TFES]) at pressures from 0.80 to 36.80 MPa. Measured data show that pressure, temperature, and characteristics of cation of ILs ([EMIM], [BMIM], and [BNMIM]) affect its CO2 solubility. We determined the CO2 solubility of fixed CO2 mole fractions for three ionic liquids. Among the three ILs, CO2 solubility intensity was ranked [BMIM][TFES] > [EMIM][TFES] ≈ [BNMIM][TFES]. The Peng–Robinson equation of state, the conventional van der Waals one-fluid mixing rule, and the modified Lydersen–Joback–Reid method were used to correlate the experimental data. The average absolute deviations of pressure were 0.0164 for CO2 + [EMIM][TFES], 0.0361 for CO2 + [BMIM][TFES], and 0.0251 for CO2 + [BNMIM][TFES] systems.
中文翻译:

在1,1,2,2-四氟乙烷磺酸根阴离子型离子液体中的CO 2溶解度:[EMIM] [TFES],[BMIM] [TFES]和[BNMIM] [TFES]
二氧化碳(CO 2)在三种不同离子液体(IL)中的溶解度:1-乙基-3-甲基咪唑鎓1,1,2,2-四氟乙烷磺酸盐([EMIM] [TFES]),1-丁基-3-甲基咪唑鎓测量了1,2,2,4-四氟乙烷磺酸盐([BMIM] [TFES])和1,1,2,2-四氟乙烷磺酸1-苄基-3-甲基咪唑鎓([BNMIM] [TFES])。在303.2至373.2 K的温度([BNMIM] [TFES]为323.2至373.2 K)的温度下,在0.80至36.80 MPa的压力下进行测量。实测数据表明,IL的压力,温度和阳离子特性([EMIM],[BMIM]和[BNMIM])会影响其CO 2溶解度。我们确定了三种离子液体的固定CO 2摩尔分数的CO 2溶解度。在三个IL中,CO 2溶解度强度的等级为[BMIM] [TFES]> [EMIM] [TFES]≈[BNMIM] [TFES]。使用了Peng-Robinson状态方程,常规的Van der Waals一流体混合法则和改进的Lydersen-Joback-Reid方法来关联实验数据。对于CO 2 + [EMIM] [TFES],平均压力绝对偏差为0.0164,对于CO 2 + [BMIM] [TFES]为0.0361,对于CO 2 + [BNMIM] [TFES]系统为0.0251。
更新日期:2020-01-22
中文翻译:

在1,1,2,2-四氟乙烷磺酸根阴离子型离子液体中的CO 2溶解度:[EMIM] [TFES],[BMIM] [TFES]和[BNMIM] [TFES]
二氧化碳(CO 2)在三种不同离子液体(IL)中的溶解度:1-乙基-3-甲基咪唑鎓1,1,2,2-四氟乙烷磺酸盐([EMIM] [TFES]),1-丁基-3-甲基咪唑鎓测量了1,2,2,4-四氟乙烷磺酸盐([BMIM] [TFES])和1,1,2,2-四氟乙烷磺酸1-苄基-3-甲基咪唑鎓([BNMIM] [TFES])。在303.2至373.2 K的温度([BNMIM] [TFES]为323.2至373.2 K)的温度下,在0.80至36.80 MPa的压力下进行测量。实测数据表明,IL的压力,温度和阳离子特性([EMIM],[BMIM]和[BNMIM])会影响其CO 2溶解度。我们确定了三种离子液体的固定CO 2摩尔分数的CO 2溶解度。在三个IL中,CO 2溶解度强度的等级为[BMIM] [TFES]> [EMIM] [TFES]≈[BNMIM] [TFES]。使用了Peng-Robinson状态方程,常规的Van der Waals一流体混合法则和改进的Lydersen-Joback-Reid方法来关联实验数据。对于CO 2 + [EMIM] [TFES],平均压力绝对偏差为0.0164,对于CO 2 + [BMIM] [TFES]为0.0361,对于CO 2 + [BNMIM] [TFES]系统为0.0251。






























 京公网安备 11010802027423号
京公网安备 11010802027423号