当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quinoline‐1,3‐Oxazole Hybrids: Syntheses, Anticancer Activity and Molecular Docking Studies
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-01-21 , DOI: 10.1002/slct.201903763 Shailesh R. Shah 1 , Kanubhai D. Katariya 1 , Dushyanth Reddy 2
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-01-21 , DOI: 10.1002/slct.201903763 Shailesh R. Shah 1 , Kanubhai D. Katariya 1 , Dushyanth Reddy 2
Affiliation
|
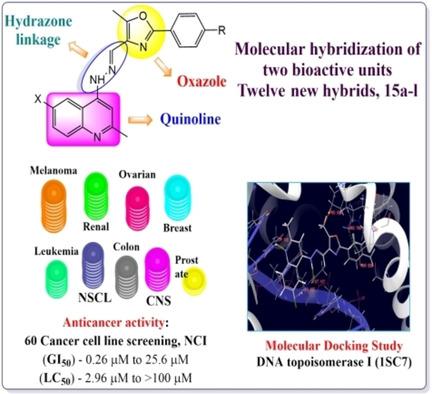
In continuation of the synthesis of potent quinoline based anticancer agents, a series of novel quinoline‐1,3‐oxazole hybrids 15 a‐l were synthesized by the condensation reaction of 2‐aryl‐5‐methyl‐1,3‐oxazole‐4‐carbaldehydes 10 a‐f and 6‐bromo/6‐chloro‐2‐methyl‐quinolin‐4‐yl‐hydrazines 14 a/b in good yield. Out of twelve, ten of the synthesized compounds were selected by the National Cancer Institute, USA for anticancer activity screening against 60 different human cancer cell lines representing nine types of cancer. Nine compounds were displayed outstanding antiproliferative activity with GI50 values ranging from 0.26 to 25.6 μM and LC50 values ranging from 2.96 μM to >100 μM. The mean MG‐MID values of GI50, TGI, and LC50 were compared with the methotrexate and four compounds 15 b, 15 d, 15 e, 15 f emerged with important GI50 <2.0 μM. Among all compounds screened, 15 d displayed the highest potency as a cytotoxic molecule. Moreover, new hybrids were also studied for molecular docking into the active binding site of DNA topoisomerase I (htopoI) to understand the binding mode and favorable interactions of active compounds into binding sites of topoisomerase enzyme.
中文翻译:

喹啉-1,3-恶唑杂化物:合成,抗癌活性和分子对接研究
在有效的喹啉的合成的基于延续的抗癌剂,一系列新的喹啉-1,3-唑杂交的15 一-升通过的2-芳基-5-甲基-1,3-唑-4-缩合反应合成-甲醛10 a - f和6-溴/ 6-氯-2-甲基-喹啉-4-基肼14 a / b,收率良好。美国国家癌症研究所从十二种化合物中选择了十种,用于筛选代表九种癌症的60种不同人类癌细胞系的抗癌活性。九种化合物显示优秀的抗增殖活性与GI 50值范围从0.26至25.6 μM和LC 50个值的范围为2.96 μ M至> 100 μ M. GI的平均MG-MID值50,TGI和LC 50与氨甲喋呤和四种化合物进行比较15 b,15 d,15 ê,15 ˚F出现了重要的GI 50 < 2.0μM。在所有筛选的化合物中,第15 天作为细胞毒性分子显示出最高的效力。此外,还研究了新的杂合体以分子对接进入DNA拓扑异构酶I(htopoI)的活性结合位点,以了解活性化合物与拓扑异构酶的结合位点的结合方式和有利的相互作用。
更新日期:2020-01-22
中文翻译:

喹啉-1,3-恶唑杂化物:合成,抗癌活性和分子对接研究
在有效的喹啉的合成的基于延续的抗癌剂,一系列新的喹啉-1,3-唑杂交的15 一-升通过的2-芳基-5-甲基-1,3-唑-4-缩合反应合成-甲醛10 a - f和6-溴/ 6-氯-2-甲基-喹啉-4-基肼14 a / b,收率良好。美国国家癌症研究所从十二种化合物中选择了十种,用于筛选代表九种癌症的60种不同人类癌细胞系的抗癌活性。九种化合物显示优秀的抗增殖活性与GI 50值范围从0.26至25.6 μM和LC 50个值的范围为2.96 μ M至> 100 μ M. GI的平均MG-MID值50,TGI和LC 50与氨甲喋呤和四种化合物进行比较15 b,15 d,15 ê,15 ˚F出现了重要的GI 50 < 2.0μM。在所有筛选的化合物中,第15 天作为细胞毒性分子显示出最高的效力。此外,还研究了新的杂合体以分子对接进入DNA拓扑异构酶I(htopoI)的活性结合位点,以了解活性化合物与拓扑异构酶的结合位点的结合方式和有利的相互作用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号