当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Enantioselective Direct Aldol Addition of Aryl Ketones to α-Fluorinated Ketones.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-01-21 , DOI: 10.1002/anie.201916129 Connor J Thomson 1 , David M Barber 2 , Darren J Dixon 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-01-21 , DOI: 10.1002/anie.201916129 Connor J Thomson 1 , David M Barber 2 , Darren J Dixon 1
Affiliation
|
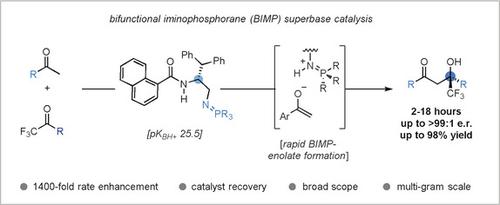
The catalytic enantioselective synthesis of α-fluorinated chiral tertiary alcohols from (hetero)aryl methyl ketones is described. The use of a bifunctional iminophosphorane (BIMP) superbase was found to facilitate direct aldol addition by providing the strong Brønsted basicity required for rapid aryl enolate formation. The new synthetic protocol is easy to perform and tolerates a broad range of functionalities and heterocycles with high enantioselectivity (up to >99:1 e.r.). Multi-gram scalability has been demonstrated along with catalyst recovery and recycling. 1 H NMR studies identified a 1400-fold rate enhancement under BIMP catalysis, compared to the prior state-of-the-art catalytic system. The utility of the aldol products has been highlighted with the synthesis of various enantioenriched building blocks and heterocycles, including 1,3-aminoalcohol, 1,3-diol, oxetane, and isoxazoline derivatives.
中文翻译:

芳基酮对α-氟化酮的催化对映选择性直接羟醛加成。
描述了由(杂)芳基甲基酮催化合成α-氟化手性叔醇的对映体。发现使用双功能亚氨基正膦(BIMP)超碱可通过提供快速形成芳基烯醇酸酯所需的强布朗斯台德碱度,促进直接添加羟醛。新的合成方案易于执行,并具有高对映选择性(高达> 99:1 er),可耐受各种功能和杂环。已经证明了多克的可扩展性以及催化剂的回收和再循环。1 H NMR研究表明,与现有技术水平的催化体系相比,BIMP催化的速率提高了1400倍。通过合成各种对映体丰富的结构单元和杂环,突出了醛醇产品的实用性,其中包括:
更新日期:2020-02-21
中文翻译:

芳基酮对α-氟化酮的催化对映选择性直接羟醛加成。
描述了由(杂)芳基甲基酮催化合成α-氟化手性叔醇的对映体。发现使用双功能亚氨基正膦(BIMP)超碱可通过提供快速形成芳基烯醇酸酯所需的强布朗斯台德碱度,促进直接添加羟醛。新的合成方案易于执行,并具有高对映选择性(高达> 99:1 er),可耐受各种功能和杂环。已经证明了多克的可扩展性以及催化剂的回收和再循环。1 H NMR研究表明,与现有技术水平的催化体系相比,BIMP催化的速率提高了1400倍。通过合成各种对映体丰富的结构单元和杂环,突出了醛醇产品的实用性,其中包括:





















































 京公网安备 11010802027423号
京公网安备 11010802027423号