当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-catalyzed allylic carbonylative coupling of alkyl zinc reagents with tert-butyl isocyanide.
Nature Communications ( IF 14.7 ) Pub Date : 2020-01-20 , DOI: 10.1038/s41467-020-14320-1 Yangyang Weng 1 , Chenhuan Zhang 1 , Zaiquan Tang 1 , Mohini Shrestha 1 , Wenyi Huang 1 , Jingping Qu 1 , Yifeng Chen 1
Nature Communications ( IF 14.7 ) Pub Date : 2020-01-20 , DOI: 10.1038/s41467-020-14320-1 Yangyang Weng 1 , Chenhuan Zhang 1 , Zaiquan Tang 1 , Mohini Shrestha 1 , Wenyi Huang 1 , Jingping Qu 1 , Yifeng Chen 1
Affiliation
|
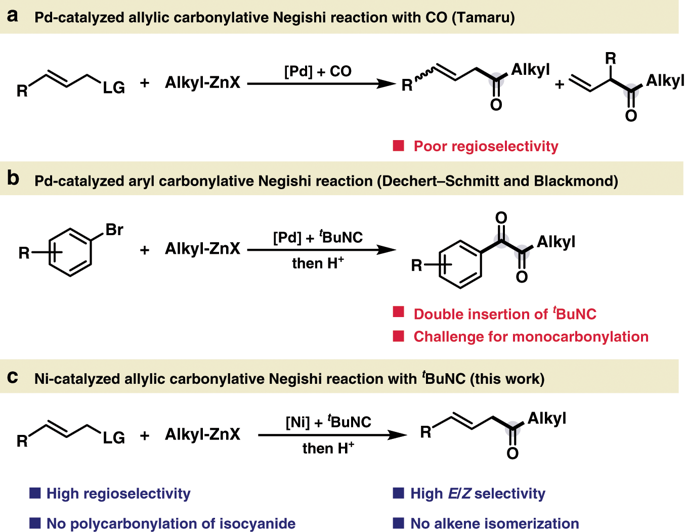
Transition metal-catalyzed carbonylation with carbon nucleophiles is one of the most prominent methods to construct ketones, which are highly versatile motifs prevalent in a variety of organic compounds. In comparison to the well-established palladium catalytic system, the nickel-catalyzed carbonylative coupling is much underdeveloped due to the strong binding affinity of CO to nickel. By leveraging easily accessible tert-butyl isocyanide as the CO surrogate, we present a nickel-catalyzed allylic carbonylative coupling with alkyl zinc reagent, allowing for the practical and straightforward preparation of synthetically important β,γ-unsaturated ketones in a linear-selective fashion with excellent trans-selectivity under mild conditions. Moreover, the undesired polycarbonylation process which is often encountered in palladium chemistry could be completely suppressed. This nickel-based method features excellent functional group tolerance, even including the active aryl iodide functionality to allow the orthogonal derivatization of β,γ-unsaturated ketones. Preliminary mechanistic studies suggest that the reaction proceeds via a π-allylnickel intermediate.
中文翻译:

烷基锌试剂与叔丁基异氰化物的镍催化烯丙基羰基偶联。
用碳亲核试剂进行的过渡金属催化的羰基化反应是构建酮的最主要方法之一,酮是多种有机化合物中普遍使用的高度通用的基序。与公认的钯催化体系相比,由于CO对镍的强结合亲和力,镍催化的羰基化偶联作用远远落后。通过利用容易获得的叔丁基异氰化物作为一氧化碳替代物,我们提出了一种镍催化的烷基烷基试剂与烯丙基羰基化偶联反应,从而可以以线性选择的方式以实用的方式直接制备合成上重要的β,γ-不饱和酮,在温和的条件下具有出色的反式选择性。此外,钯化学中经常遇到的不希望的多羰基化过程可以完全被抑制。这种基于镍的方法具有出色的官能团耐受性,甚至包括活性芳基碘官能团,可实现β,γ-不饱和酮的正交衍生。初步的机理研究表明,该反应通过π-烯丙基镍中间体进行。
更新日期:2020-01-22
中文翻译:

烷基锌试剂与叔丁基异氰化物的镍催化烯丙基羰基偶联。
用碳亲核试剂进行的过渡金属催化的羰基化反应是构建酮的最主要方法之一,酮是多种有机化合物中普遍使用的高度通用的基序。与公认的钯催化体系相比,由于CO对镍的强结合亲和力,镍催化的羰基化偶联作用远远落后。通过利用容易获得的叔丁基异氰化物作为一氧化碳替代物,我们提出了一种镍催化的烷基烷基试剂与烯丙基羰基化偶联反应,从而可以以线性选择的方式以实用的方式直接制备合成上重要的β,γ-不饱和酮,在温和的条件下具有出色的反式选择性。此外,钯化学中经常遇到的不希望的多羰基化过程可以完全被抑制。这种基于镍的方法具有出色的官能团耐受性,甚至包括活性芳基碘官能团,可实现β,γ-不饱和酮的正交衍生。初步的机理研究表明,该反应通过π-烯丙基镍中间体进行。































 京公网安备 11010802027423号
京公网安备 11010802027423号