当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural analogues of quinoline alkaloids: Straightforward route to [1,3]dioxolo[4,5‐c]quinolines with antibacterial properties
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-01-21 , DOI: 10.1002/jhet.3886 Radim Horák 1 , Kamil Kořistek 1 , Veronika Šamšulová 1 , Ludmila Slaninová 1 , Martin Grepl 1 , Lubomír Kvapil 1 , Petr Funk 1 , Pavel Hradil 1 , Miroslav Soural 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-01-21 , DOI: 10.1002/jhet.3886 Radim Horák 1 , Kamil Kořistek 1 , Veronika Šamšulová 1 , Ludmila Slaninová 1 , Martin Grepl 1 , Lubomír Kvapil 1 , Petr Funk 1 , Pavel Hradil 1 , Miroslav Soural 1
Affiliation
|
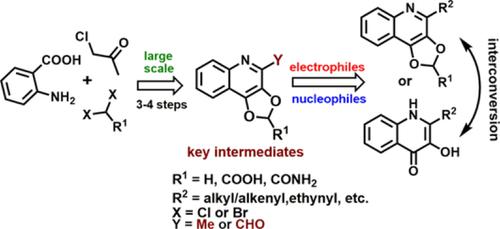
Compounds bearing [1,3]dioxolo‐quinoline scaffolds have been found in quinoline‐based natural products; the only exception is the [1,3]dioxolo[4,5‐c]quinoline moiety with a rare occurrence in both natural and synthetic derivatives. In this article, we report the preparation of diversely substituted and functionalized [1,3]dioxolo[4,5‐c]quinolines using [1,3]dioxolo[4,5‐c]quinoline‐4‐carbaldehyde (DQC) as the common intermediate. DQC was synthesized on a large scale from anthranilic acid and chloroacetone as the starting materials, with the rearrangement of acetonyl‐anthranilate as the key step. The developed method allows for the simple preparation of [1,3]dioxolo[4,5‐c]quinolines with various C2 substituents on the quinoline scaffold. Additionally, the synthetic route was successfully applied to the preparation of 3‐hydroxyquinoline‐4(1H)‐ones. The target compounds were tested against representative Gram‐positive/negative bacteria, and two derivatives exhibited submicromolar minimum inhibitory concentrations against Micrococcus luteus.
中文翻译:

喹啉生物碱的结构类似物:具有抗菌特性的[1,3] dioxolo [4,5-c]喹啉的直接合成途径
在基于喹啉的天然产物中发现了带有[1,3]二氧杂喹啉骨架的化合物。唯一的例外是[1,3] dioxolo [4,5- c ]喹啉部分,在天然和合成衍生物中均很少见。在本文中,我们报道了制备的多样化取代的和官能化的[1,3]二氧杂环戊烯并[4,5- c ^ ]喹啉使用[1,3]二氧杂环戊烯并[4,5-c]喹啉-4-甲醛(DQC)作为常见的中间体。DQC以邻氨基苯甲酸和氯丙酮为起始原料进行大规模合成,其中乙酰基邻氨基苯甲酸酯的重排是关键步骤。所开发的方法可以轻松制备[1,3] dioxolo [4,5- c在喹啉支架上具有各种C2取代基的喹啉。此外,合成路线已成功地用于制备3-羟基喹啉-4(1 H)-酮。对目标化合物进行了针对代表性革兰氏阳性/阴性细菌的测试,并且两种衍生物均表现出对微球菌微球菌的最小微摩尔抑制浓度。
更新日期:2020-01-22
中文翻译:

喹啉生物碱的结构类似物:具有抗菌特性的[1,3] dioxolo [4,5-c]喹啉的直接合成途径
在基于喹啉的天然产物中发现了带有[1,3]二氧杂喹啉骨架的化合物。唯一的例外是[1,3] dioxolo [4,5- c ]喹啉部分,在天然和合成衍生物中均很少见。在本文中,我们报道了制备的多样化取代的和官能化的[1,3]二氧杂环戊烯并[4,5- c ^ ]喹啉使用[1,3]二氧杂环戊烯并[4,5-c]喹啉-4-甲醛(DQC)作为常见的中间体。DQC以邻氨基苯甲酸和氯丙酮为起始原料进行大规模合成,其中乙酰基邻氨基苯甲酸酯的重排是关键步骤。所开发的方法可以轻松制备[1,3] dioxolo [4,5- c在喹啉支架上具有各种C2取代基的喹啉。此外,合成路线已成功地用于制备3-羟基喹啉-4(1 H)-酮。对目标化合物进行了针对代表性革兰氏阳性/阴性细菌的测试,并且两种衍生物均表现出对微球菌微球菌的最小微摩尔抑制浓度。































 京公网安备 11010802027423号
京公网安备 11010802027423号