当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Size-Switchable Nanoparticles with Self-Destructive and Tumor Penetration Characteristics for Site-Specific Phototherapy of Cancer.
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-02-03 , DOI: 10.1021/acsami.9b21525 Kewei Wang 1 , Yalan Tu 1, 2 , Wang Yao 1 , Qingyu Zong 3 , Xuan Xiao 4 , Rui-Meng Yang 1 , Xin-Qing Jiang 1 , Youyong Yuan 1, 5, 6
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-02-03 , DOI: 10.1021/acsami.9b21525 Kewei Wang 1 , Yalan Tu 1, 2 , Wang Yao 1 , Qingyu Zong 3 , Xuan Xiao 4 , Rui-Meng Yang 1 , Xin-Qing Jiang 1 , Youyong Yuan 1, 5, 6
Affiliation
|
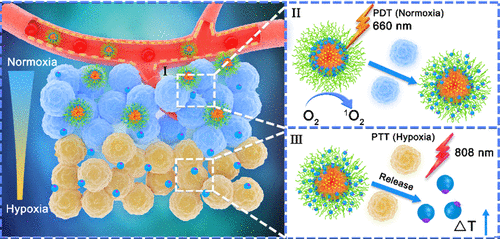
The normoxic and hypoxic microenvironments in solid tumors cause cancer cells to show different sensitivities to various treatments. Therefore, it is essential to develop different therapeutic modalities based on the tumor microenvironment. In this study, we designed size-switchable nanoparticles with self-destruction and tumor penetration characteristics for site-specific phototherapy of cancer. This was achieved by photodynamic therapy in the perivascular normoxic microenvironment due to high local oxygen concentrations and photothermal therapy (PTT) in the hypoxic microenvironment, which are not in proximity to blood vessels due to a lack of effective approaches for heat transfer. In brief, a poly(amidoamine) dendrimer with photothermal agent indocyanine green (PAMAM-ICG) was conjugated to the amphiphilic polymer through a singlet oxygen-responsive thioketal linker and then loaded with photosensitizer chlorin e6 (Ce6) to construct a nanotherapy platform (denoted as SNPICG/Ce6). After intravenous injection, SNPICG/Ce6 was accumulated at the perivascular sites of the tumor. The singlet oxygen produced by Ce6 can ablate the tumor cells in the normoxic microenvironment and simultaneously cleave the thioketal linker, allowing the release of small PAMAM-ICGs with improved tumor penetration for PTT in the hypoxic microenvironment. This tailored site-specific phototherapy in normoxic and hypoxic microenvironments provides an effective strategy for cancer therapy.
中文翻译:

具有自毁和肿瘤穿透特性的尺寸可转换纳米颗粒,用于癌症的特定部位光疗。
实体瘤中的常氧和低氧微环境使癌细胞对各种治疗方法表现出不同的敏感性。因此,基于肿瘤微环境开发不同的治疗方式是至关重要的。在这项研究中,我们设计了具有自毁性和肿瘤穿透特性的尺寸可切换纳米粒子,用于癌症的特定部位光疗。这是通过在血管周围常氧微环境中进行光动力疗法而实现的,这是由于局部氧浓度高而在缺氧微环境中进行光热疗法(PTT)所致,由于缺乏有效的传热方法,这种疗法无法靠近血管。简单来说,将具有光热剂吲哚花青绿(PAMAM-ICG)的聚(酰胺基胺)树状大分子通过单线态氧响应性硫缩酮连接物与两亲聚合物偶联,然后加载光敏剂二氢卟酚e6(Ce6)以构建纳米治疗平台(表示为SNPICG / Ce6)。静脉注射后,SNPICG / Ce6积聚在肿瘤的血管周围部位。Ce6产生的单线态氧可以消融常氧微环境中的肿瘤细胞,并同时裂解硫缩酮连接基,从而允许释放较小的PAMAM-ICG,从而改善低氧微环境中PTT的肿瘤渗透性。在常氧和低氧的微环境中量身定制的针对特定地点的光疗为癌症治疗提供了有效的策略。
更新日期:2020-02-03
中文翻译:

具有自毁和肿瘤穿透特性的尺寸可转换纳米颗粒,用于癌症的特定部位光疗。
实体瘤中的常氧和低氧微环境使癌细胞对各种治疗方法表现出不同的敏感性。因此,基于肿瘤微环境开发不同的治疗方式是至关重要的。在这项研究中,我们设计了具有自毁性和肿瘤穿透特性的尺寸可切换纳米粒子,用于癌症的特定部位光疗。这是通过在血管周围常氧微环境中进行光动力疗法而实现的,这是由于局部氧浓度高而在缺氧微环境中进行光热疗法(PTT)所致,由于缺乏有效的传热方法,这种疗法无法靠近血管。简单来说,将具有光热剂吲哚花青绿(PAMAM-ICG)的聚(酰胺基胺)树状大分子通过单线态氧响应性硫缩酮连接物与两亲聚合物偶联,然后加载光敏剂二氢卟酚e6(Ce6)以构建纳米治疗平台(表示为SNPICG / Ce6)。静脉注射后,SNPICG / Ce6积聚在肿瘤的血管周围部位。Ce6产生的单线态氧可以消融常氧微环境中的肿瘤细胞,并同时裂解硫缩酮连接基,从而允许释放较小的PAMAM-ICG,从而改善低氧微环境中PTT的肿瘤渗透性。在常氧和低氧的微环境中量身定制的针对特定地点的光疗为癌症治疗提供了有效的策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号