当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ionizable Lipid Nanoparticle-Mediated mRNA Delivery for Human CAR T Cell Engineering.
Nano Letters ( IF 9.6 ) Pub Date : 2020-02-05 , DOI: 10.1021/acs.nanolett.9b04246 Margaret M Billingsley 1 , Nathan Singh 2, 3 , Pranali Ravikumar 2 , Rui Zhang 1 , Carl H June 4, 5 , Michael J Mitchell 1, 4, 6, 7, 8
Nano Letters ( IF 9.6 ) Pub Date : 2020-02-05 , DOI: 10.1021/acs.nanolett.9b04246 Margaret M Billingsley 1 , Nathan Singh 2, 3 , Pranali Ravikumar 2 , Rui Zhang 1 , Carl H June 4, 5 , Michael J Mitchell 1, 4, 6, 7, 8
Affiliation
|
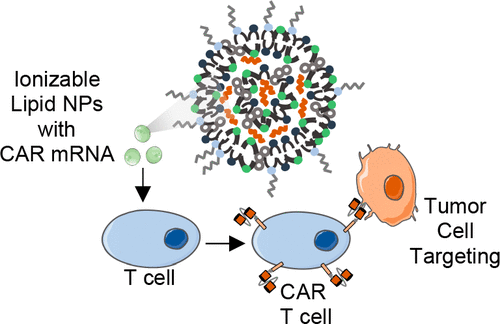
Chimeric antigen receptor (CAR) T cell therapy relies on the ex vivo manipulation of patient T cells to create potent, cancer-targeting therapies, shown to be capable of inducing remission in patients with acute lymphoblastic leukemia and large B cell lymphoma. However, current CAR T cell engineering methods use viral delivery vectors, which induce permanent CAR expression and could lead to severe adverse effects. Messenger RNA (mRNA) has been explored as a promising strategy for inducing transient CAR expression in T cells to mitigate the adverse effects associated with viral vectors, but it most commonly requires electroporation for T cell mRNA delivery, which can be cytotoxic. Here, ionizable lipid nanoparticles (LNPs) were designed for ex vivo mRNA delivery to human T cells. A library of 24 ionizable lipids was synthesized, formulated into LNPs, and screened for luciferase mRNA delivery to Jurkat cells, revealing seven formulations capable of enhanced mRNA delivery over lipofectamine. The top-performing LNP formulation, C14-4, was selected for CAR mRNA delivery to primary human T cells. This platform induced CAR expression at levels equivalent to electroporation, with substantially reduced cytotoxicity. CAR T cells engineered via C14-4 LNP treatment were then compared to electroporated CAR T cells in a coculture assay with Nalm-6 acute lymphoblastic leukemia cells, and both CAR T cell engineering methods elicited potent cancer-killing activity. These results demonstrate the ability of LNPs to deliver mRNA to primary human T cells to induce functional protein expression, and indicate the potential of LNPs to enhance mRNA-based CAR T cell engineering methods.
中文翻译:

用于人CAR T细胞工程的可电离的脂质纳米颗粒介导的mRNA传递。
嵌合抗原受体(CAR)T细胞疗法依赖于对患者T细胞的离体操作,以产生有效的,靶向癌症的疗法,该疗法被证明能够诱导急性淋巴细胞白血病和大B细胞淋巴瘤患者缓解。然而,当前的CAR T细胞工程方法使用病毒递送载体,其诱导永久性CAR表达并可能导致严重的不良反应。信使RNA(mRNA)已被探索为在T细胞中诱导瞬时CAR表达以减轻与病毒载体相关的不利影响的一种有前途的策略,但是最常见的方法是电穿孔以递送T细胞mRNA,这可能具有细胞毒性。在这里,可电离脂质纳米颗粒(LNPs)被设计用于离体mRNA传递到人类T细胞。合成了24种可离子化脂质的文库,将其配制成LNP,并筛选将荧光素酶mRNA递送至Jurkat细胞的过程,揭示了七种能够比脂质转染胺增强mRNA递送的制剂。选择了性能最高的LNP制剂C14-4用于将CAR mRNA递送至原代人T细胞。该平台以与电穿孔相当的水平诱导CAR表达,细胞毒性大大降低。然后,在与Nalm-6急性淋巴细胞白血病细胞共培养的实验中,将通过C14-4 LNP处理工程化的CAR T细胞与电穿孔的CAR T细胞进行了比较,两种CAR T细胞工程方法均具有有效的杀癌活性。这些结果证明了LNP将mRNA递送至原代人T细胞以诱导功能性蛋白表达的能力,并表明了LNP增强基于mRNA的CAR T细胞工程方法的潜力。并筛选了荧光素酶mRNA传递到Jurkat细胞的方法,揭示了7种能够比lipofectamine增强mRNA传递的制剂。选择性能最高的LNP制剂C14-4用于将CAR mRNA递送至原代人T细胞。该平台以与电穿孔相当的水平诱导CAR表达,细胞毒性大大降低。然后,在与Nalm-6急性淋巴细胞白血病细胞共培养的实验中,将通过C14-4 LNP处理工程化的CAR T细胞与电穿孔的CAR T细胞进行了比较,两种CAR T细胞工程方法均具有有效的杀癌活性。这些结果证明了LNP将mRNA递送至原代人T细胞以诱导功能性蛋白表达的能力,并表明了LNP增强基于mRNA的CAR T细胞工程方法的潜力。并筛选了荧光素酶mRNA传递到Jurkat细胞的方法,揭示了7种能够比lipofectamine增强mRNA传递的制剂。选择性能最高的LNP制剂C14-4用于将CAR mRNA递送至原代人T细胞。该平台以与电穿孔相当的水平诱导CAR表达,细胞毒性大大降低。然后,在与Nalm-6急性淋巴细胞白血病细胞共培养的实验中,将通过C14-4 LNP处理工程化的CAR T细胞与电穿孔的CAR T细胞进行了比较,两种CAR T细胞工程方法均具有有效的杀癌活性。这些结果证明了LNP将mRNA递送至原代人T细胞以诱导功能性蛋白表达的能力,并表明了LNP增强基于mRNA的CAR T细胞工程方法的潜力。
更新日期:2020-02-06
中文翻译:

用于人CAR T细胞工程的可电离的脂质纳米颗粒介导的mRNA传递。
嵌合抗原受体(CAR)T细胞疗法依赖于对患者T细胞的离体操作,以产生有效的,靶向癌症的疗法,该疗法被证明能够诱导急性淋巴细胞白血病和大B细胞淋巴瘤患者缓解。然而,当前的CAR T细胞工程方法使用病毒递送载体,其诱导永久性CAR表达并可能导致严重的不良反应。信使RNA(mRNA)已被探索为在T细胞中诱导瞬时CAR表达以减轻与病毒载体相关的不利影响的一种有前途的策略,但是最常见的方法是电穿孔以递送T细胞mRNA,这可能具有细胞毒性。在这里,可电离脂质纳米颗粒(LNPs)被设计用于离体mRNA传递到人类T细胞。合成了24种可离子化脂质的文库,将其配制成LNP,并筛选将荧光素酶mRNA递送至Jurkat细胞的过程,揭示了七种能够比脂质转染胺增强mRNA递送的制剂。选择了性能最高的LNP制剂C14-4用于将CAR mRNA递送至原代人T细胞。该平台以与电穿孔相当的水平诱导CAR表达,细胞毒性大大降低。然后,在与Nalm-6急性淋巴细胞白血病细胞共培养的实验中,将通过C14-4 LNP处理工程化的CAR T细胞与电穿孔的CAR T细胞进行了比较,两种CAR T细胞工程方法均具有有效的杀癌活性。这些结果证明了LNP将mRNA递送至原代人T细胞以诱导功能性蛋白表达的能力,并表明了LNP增强基于mRNA的CAR T细胞工程方法的潜力。并筛选了荧光素酶mRNA传递到Jurkat细胞的方法,揭示了7种能够比lipofectamine增强mRNA传递的制剂。选择性能最高的LNP制剂C14-4用于将CAR mRNA递送至原代人T细胞。该平台以与电穿孔相当的水平诱导CAR表达,细胞毒性大大降低。然后,在与Nalm-6急性淋巴细胞白血病细胞共培养的实验中,将通过C14-4 LNP处理工程化的CAR T细胞与电穿孔的CAR T细胞进行了比较,两种CAR T细胞工程方法均具有有效的杀癌活性。这些结果证明了LNP将mRNA递送至原代人T细胞以诱导功能性蛋白表达的能力,并表明了LNP增强基于mRNA的CAR T细胞工程方法的潜力。并筛选了荧光素酶mRNA传递到Jurkat细胞的方法,揭示了7种能够比lipofectamine增强mRNA传递的制剂。选择性能最高的LNP制剂C14-4用于将CAR mRNA递送至原代人T细胞。该平台以与电穿孔相当的水平诱导CAR表达,细胞毒性大大降低。然后,在与Nalm-6急性淋巴细胞白血病细胞共培养的实验中,将通过C14-4 LNP处理工程化的CAR T细胞与电穿孔的CAR T细胞进行了比较,两种CAR T细胞工程方法均具有有效的杀癌活性。这些结果证明了LNP将mRNA递送至原代人T细胞以诱导功能性蛋白表达的能力,并表明了LNP增强基于mRNA的CAR T细胞工程方法的潜力。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号