当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Controlling the Oxidation State of Cu Electrode and Reaction Intermediates for Electrochemical CO2 Reduction to Ethylene
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-20 , DOI: 10.1021/jacs.9b11126
Tsu-Chin Chou, Chiao-Chun Chang, Hung-Ling Yu, Wen-Yueh Yu, Chung-Li Dong, Juan-Jesús Velasco-Vélez, Cheng-Hao Chuang, Li-Chyong Chen, Jyh-Fu Lee, Jin-Ming Chen, Heng-Liang Wu
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-01-20 , DOI: 10.1021/jacs.9b11126
Tsu-Chin Chou, Chiao-Chun Chang, Hung-Ling Yu, Wen-Yueh Yu, Chung-Li Dong, Juan-Jesús Velasco-Vélez, Cheng-Hao Chuang, Li-Chyong Chen, Jyh-Fu Lee, Jin-Ming Chen, Heng-Liang Wu
|
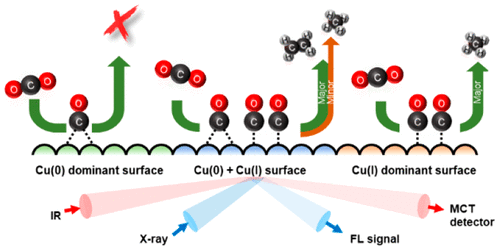
Understanding the role of oxidation state of Cu surface and surface-adsorbed intermediate species in electrochemical CO2 reduction is crucial for the development of selective CO2-to-fuel electrocatalysts. In this study, the electrochemical CO2 reduction mechanism over the Cu catalysts with various oxidation states was studied by using in situ surface-enhanced infrared absorption spectroscopy (SEIRAS), in situ soft X-ray absorption spectroscopy (Cu L-edge) and on-line gas chromatography measurements. The atop-adsorbed CO (COatop) intermediate is obtained on the electrodeposited Cu surface which primarily has the oxidation state of Cu(I). COatop is further reduced, followed by the formation of C1 product such as CH4. The residual bridge-adsorbed CO (CObridge) is formed on the as-prepared Cu surface with Cu(0) which inhibits hydrocarbon formation. In contrast, the CV-treated Cu electrode prepared by oxidizing the as-prepared Cu surface contains different amount of Cu(I) and Cu(0) states. The major theme of this work is that in situ SEIRAS results show the coexistence of COatop and CObridge as the reaction intermediates during CO2 reduction and the selectivity of CO2-to-ethylene conversion is further enhanced in the CV-treated Cu electrode. The Cu catalysts modulated by electrochemical method exhibit different oxidation states and reaction intermediates as well as the electrocatalytic properties.
更新日期:2020-01-20

































 京公网安备 11010802027423号
京公网安备 11010802027423号